L-serine compositions, methods and uses for treating neurodegenerative diseases and disorders
a neurodegenerative disease and composition technology, applied in the field of lserine compositions, methods and uses for treating neurodegenerative diseases and disorders, to achieve the effect of preventing, reducing or inhibiting the onset, severity, frequency or duration of one or more symptoms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0103]This example describes various materials and methods.
Materials & Methods
[0104]MRC-5 cells were from American Tissue and Cell Culture (Virginia, USA). SH-SY5Y cells were from the European Collection of Cell Culture (ECACC). 3H-BMAA (80 Ci / mmol, 0.5 mCi / mL) was obtained from American Radiolabeled Chemicals. Dulbecco's modification of Eagle's minimum essential medium (DMEM) and HAMS F12 were from JRH biosciences, (Lenexa, Kansans, USA). BMAA, dithiothreitol, L-serine, D-serine, acridine orange, ethidium bromide, cycloheximide and SDS were from Sigma Chemical Co. (Sigma-Aldrich, Castle Hill, NSW, Australia). BCA protein reagent was from Pierce Biotechnology (Rockford, Ill., USA). BD Pharminigen™ Annexin V-FITC apoptosis detection kit was from BD Biosciences (Sydney Australia). Water was from a Milli Q 4 stage system (Millipore-Waters, Lane Cove, NSW, Australia). All HPLC equipment was supplied by the Shimadzu Corporation (Kyoto, Japan) except the column (Nova-Pak® C18 4 μM 3.9×300...
example 2
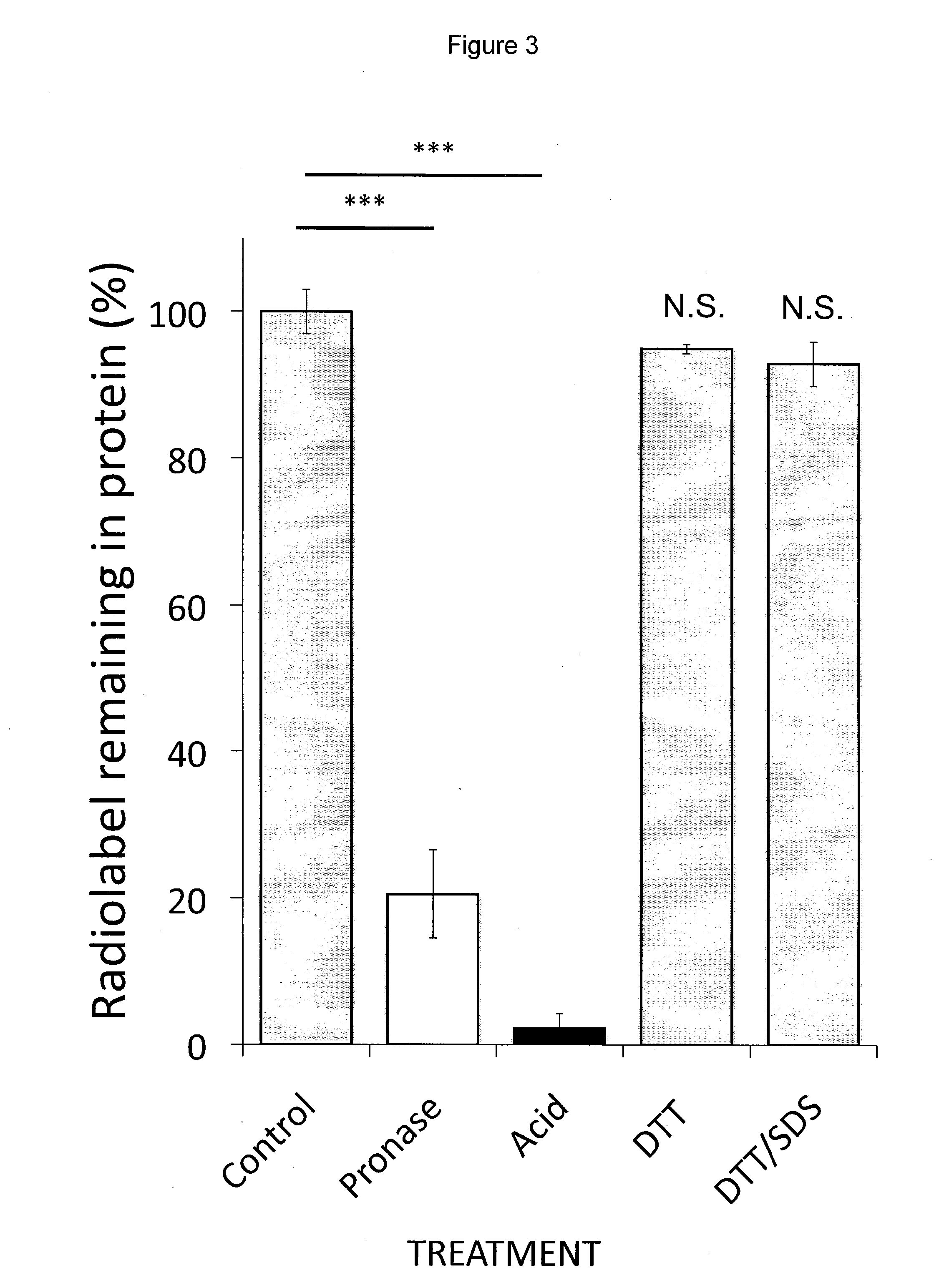
[0120]This example describes data indicating that BMAA is incorporated into proteins, which can lead to protein misfolding / aggregation, and which incorporation and misfolding / aggregation is inhibited by L-serine.
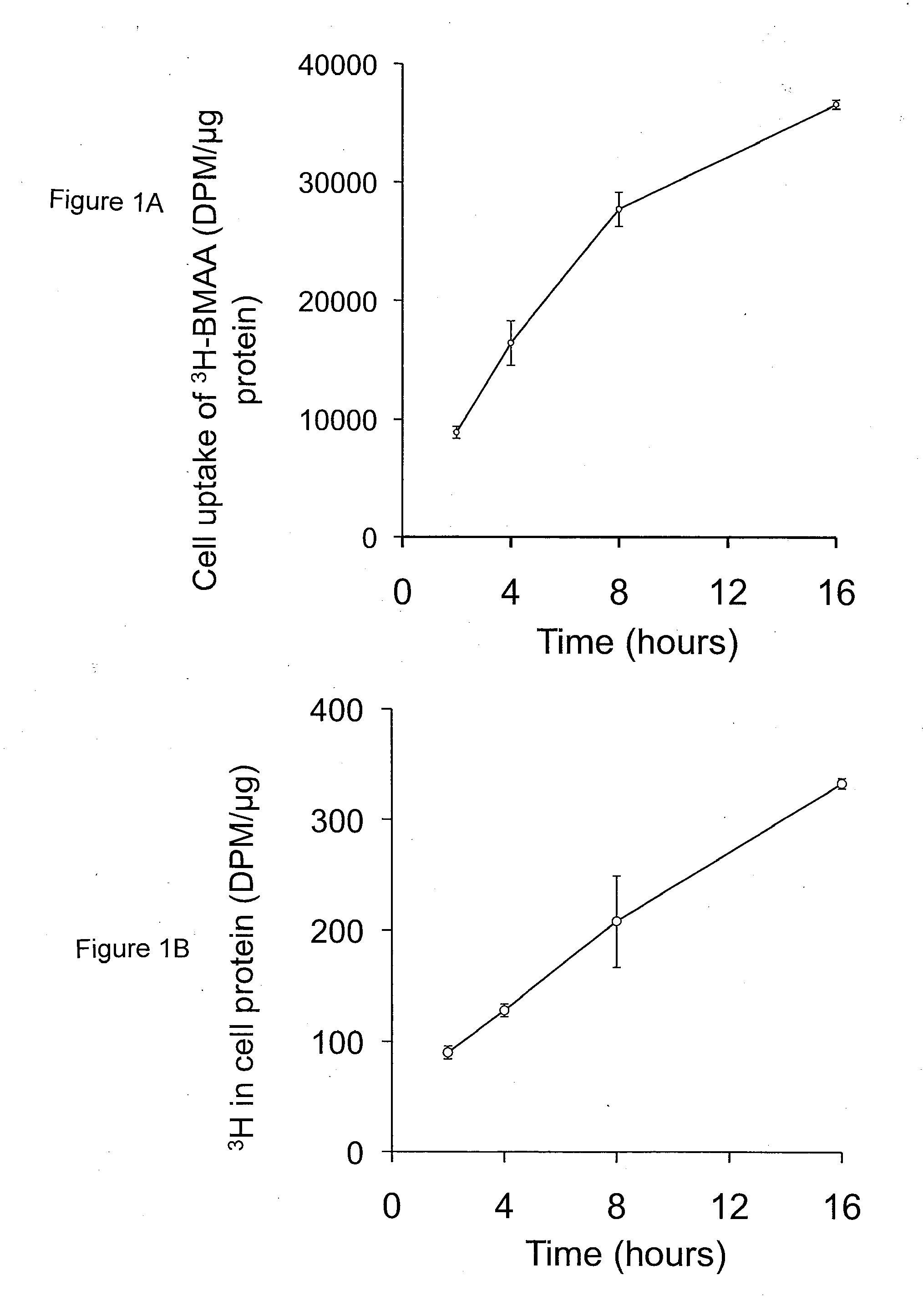
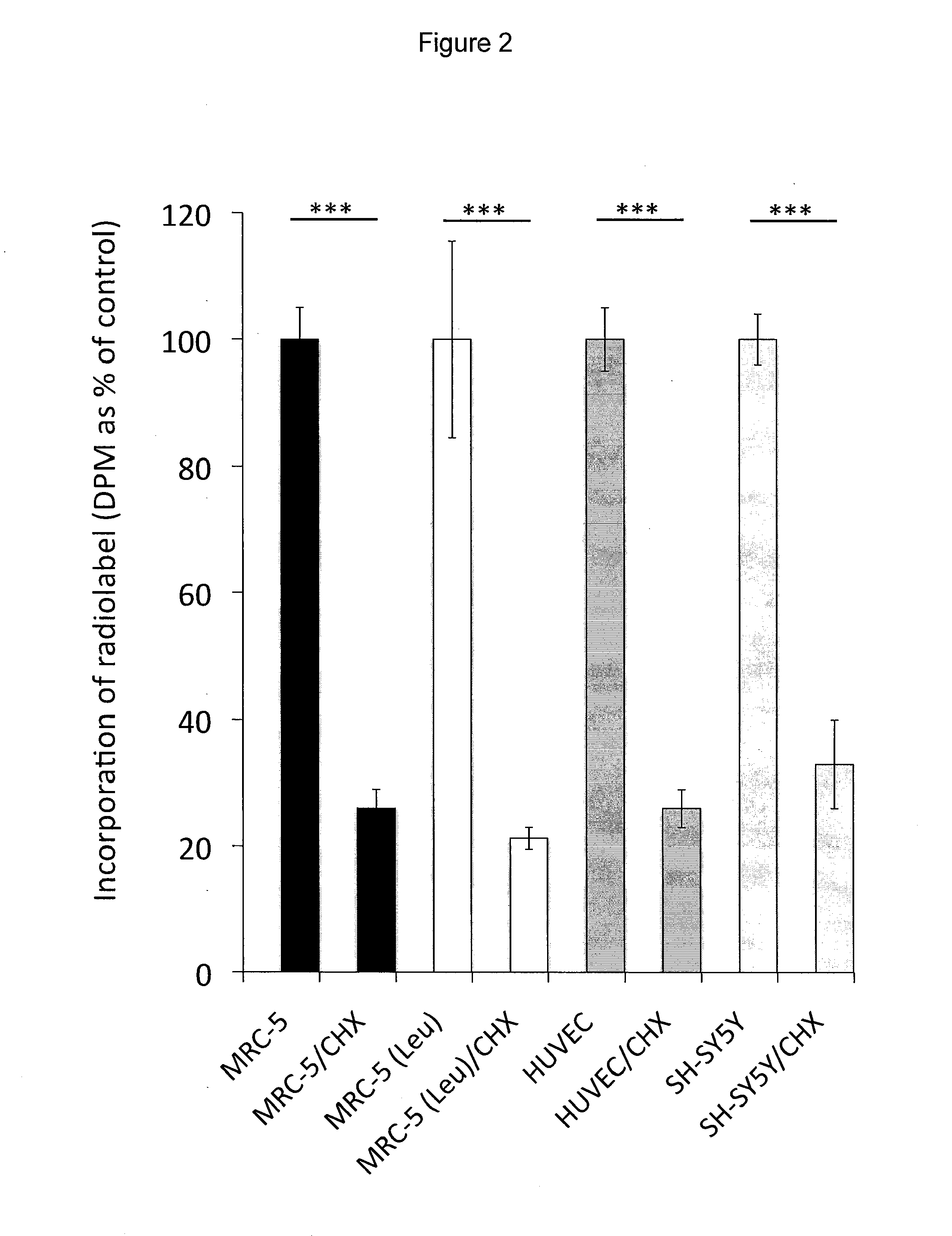
[0121]Incubation of human MRC-5 fibroblasts with 3H-BMAA in culture medium depleted in amino acids resulted in a time-dependent increase in radiolabel in cell lysates (FIG. 1A), a proportion of which was associated with cell proteins (FIG. 1B). Co-incubation of MRC-5 cells with 3H-BMAA along with the protein synthesis inhibitor CHX significantly reduced the amount of radiolabel in the protein fraction (FIG. 2). CHX inhibited incorporation of the protein amino acid 3H-leucine into proteins to the same extent as 3H-BMAA (FIG. 2) suggesting that 3H-BMAA was incorporated into proteins by a protein synthesis-dependent mechanism. 3H-BMAA was also found to be protein associated after incubation with human primary endothelial cells (HUVEC) and human neuroblastoma cells (SH-SY5Y), th...
example 3
[0128]This example describes data indicating that L-Serine rescues Drosophila melanogaster (fruit flies) from BMAA induced mortality, and additional useful models.
[0129]APP is evolutionarily conserved from invertebrates to vertebrates. This allows research to be performed on APP in Drosophila fruit flies, to study human diseases such as Alzheimer's disease. The Drosophila model was used to characterize how BMAA influences the production of the APP-derived fragments, especially the amyloidogenic fragments.
The Drosophila melanogaster (fruit fly) insect model is a convenient invertebrate system to measure important features of a human disease, including plaque formation, memory loss, social interactions, and the overall neuronal structure of a model organism. Fruit flies also engineered to produce extra human Aβ will be fed BMAA and the resulting increase or decrease in plaque inducing Aβ will be measured. Flies are an excellent model because their genetic characteristics have been wel...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pH | aaaaa | aaaaa |
| vaporizer temperature | aaaaa | aaaaa |
| nebulizing pressure | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com