Intracorporeal Component for a Percutaneous Device
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0061]The present embodiments represent currently the best ways known to the applicant of putting the invention into practice. But they are not the only ways in which this can be achieved. They are illustrated, and they will now be described, by way of example only.
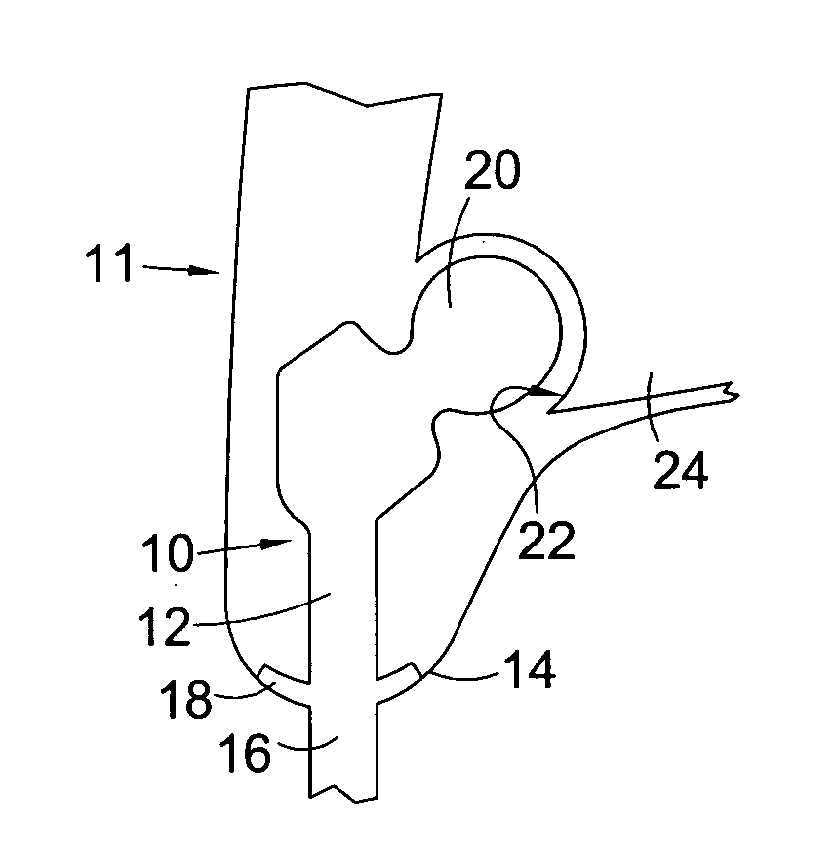
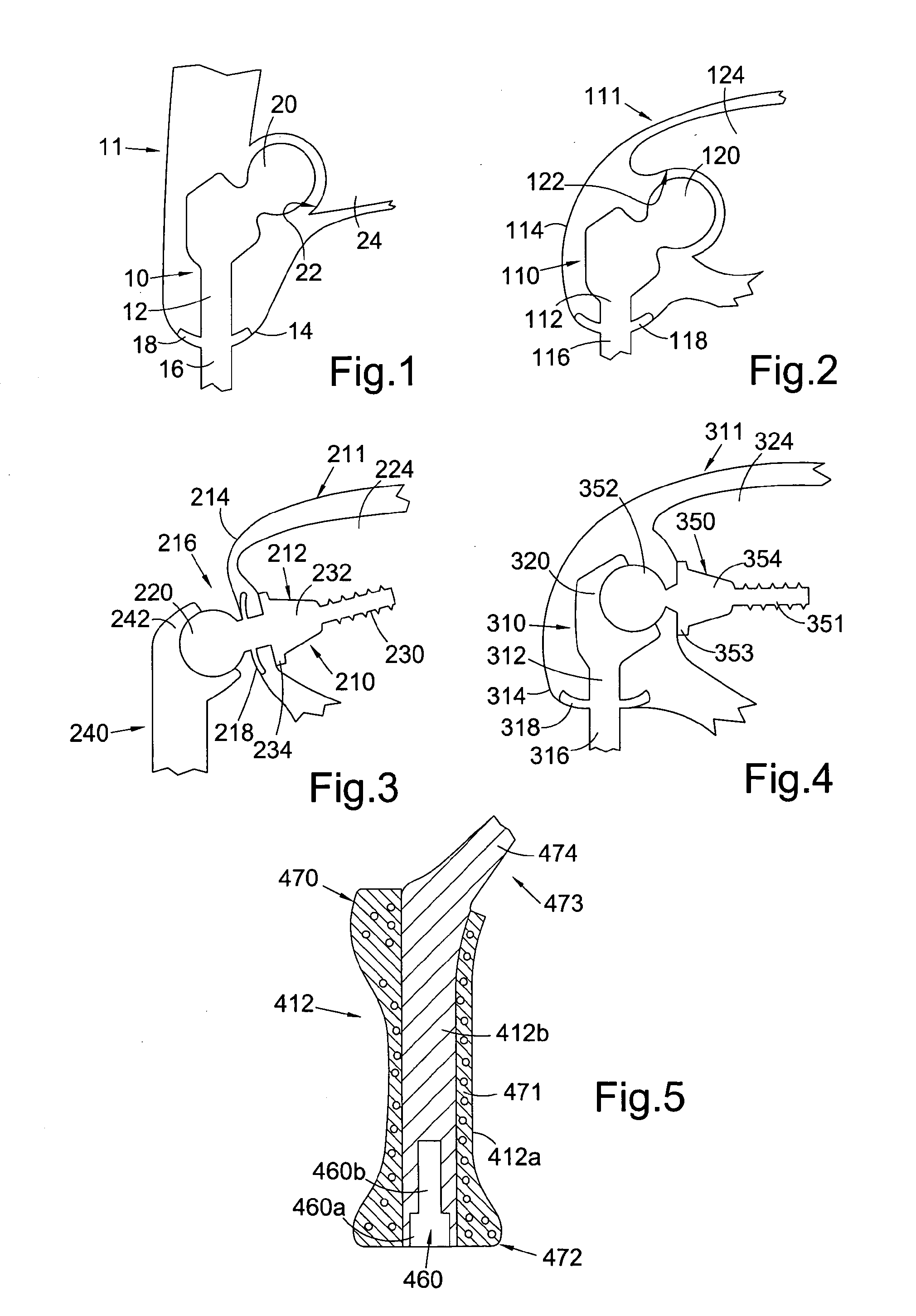
[0062]Referring to FIG. 1, this shows a percutaneous device 10 installed in a subject 11. The percutaneous device 10 has an intracorporeal or subcutaneous portion 12 which resides under the skin 14 when the device is installed and an extracorporeal or external portion 16 which extends from the skin when the device is installed. The device may be made up from separate components connected together, or the components may be formed integrally.
[0063]In FIG. 1, the percutaneous device 10 is shown installed at the subject's hip. The device 10 has a ball component 20 at its proximal end when installed which is received in and articulates with the subject's natural hip socket (the acetabulum) 22 in the subject's pelvis 24. Altern...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com