Remedy for Chronic Inflammation and Antibody to be Used Therein
a chronic inflammation and antibody technology, applied in the field of chronic inflammation drugs, can solve the problems of inconsistent effects of conventionally-known anti-tenascin-c antibodies against inflammation, no details given, and no clear finding regarding their relationship with chronic inflammation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0045]A peptide having the amino acid sequence of SEQ ID NO: 3 was synthesized by a usual method. The synthesized peptide as hapten was fused with KLH, and the obtained fusion peptide was used as an antigenic peptide. Immunization was conducted in accordance with a usual method using rabbits. The mixture including a target antibody obtained was purified by a usual method.
[0046]In the manner as above, three kinds (No. 1 to No. 3) of polyclonal antibodies (anti-human TNIIIA2 antibodies) against the human TNIIIA2 were obtained.
[0047]
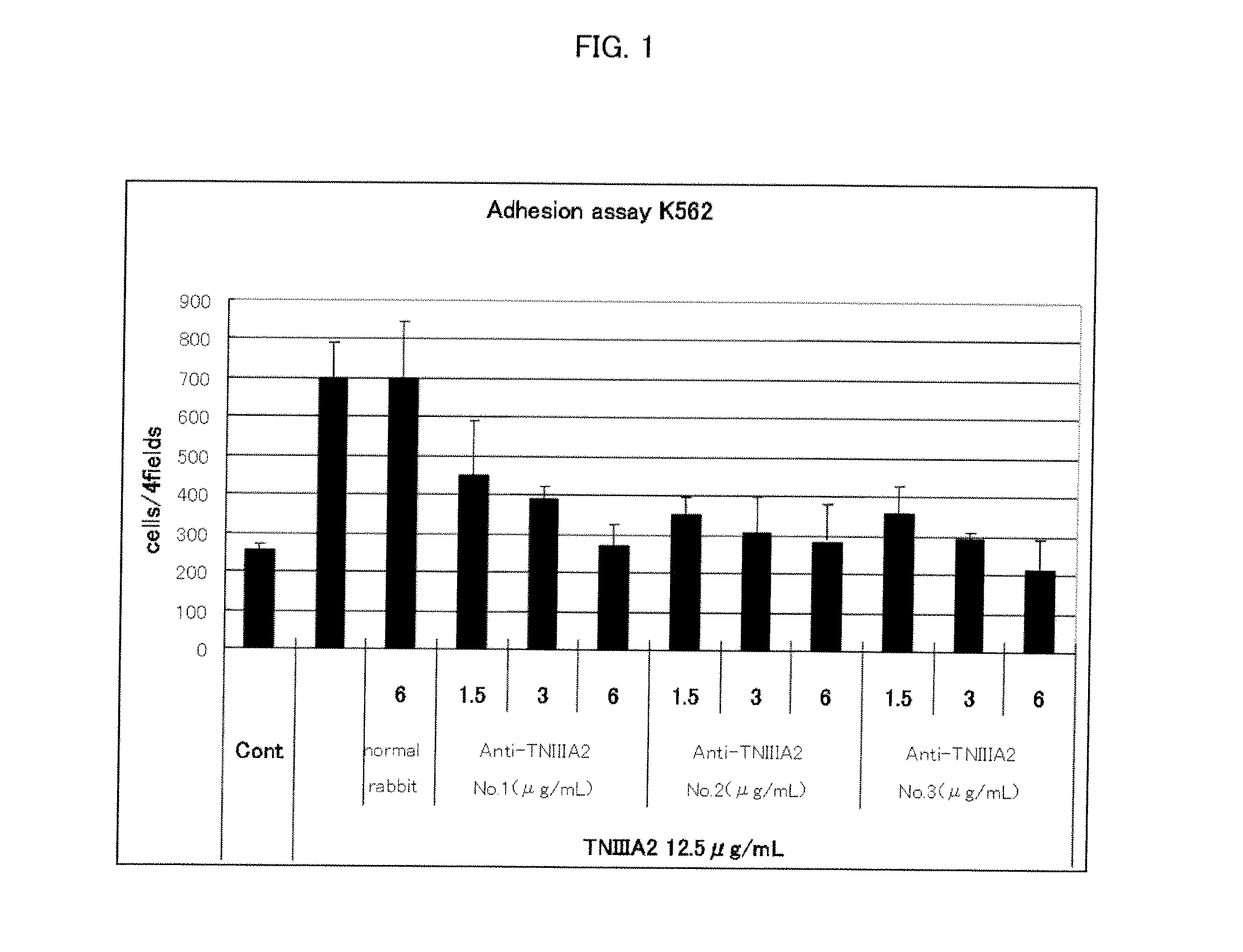
[0048]Antibody activity of the anti-human TNIIIA2 polyclonal antibodies obtained above were evaluated as follows by using, as indicators, their inhibition effects against cell adhesion induced by the human TNIIIA2. The results are shown in Table 1.
[0049]Using RPMI 1640 (20% FBS added) as a cell culture medium, KOP 2.16 cells (a mouse bone marrow-derived vascular endothelial cell line) were seeded in each well of a 96-hole plate at a density of 5×104 cells / 2...
example 3
[0069]The effect of the anti-human TNIIIA2 antibody on changes in viable macrophage cell counts over time in the presence of human tenascin-C was evaluated as follows.
[0070]THP-1 cells were cultured in a complete medium, to which PMA was added at a concentration of 10 ng / mL, at 37° C. and 5% CO2 for 24 hours to be induced to differentiate into macrophages (hereinafter, the differentiated THP-1 is referred to as “PMA-THP-1”).
[0071]Into each well of a 96-hole plate, fibronectin (FN) was added at a concentration of 5 μg / mL, and the plate was incubated at 37° C. for 1 hour. The plate was blocked by adding BSA at a concentration of 2.5 mg / mL and incubating at 37° C. for 1 hour, and washed three times with PBS to obtain a plate with FN-coated each well.
[0072]Into the FN-coated wells, an RPMI 1640 (serum-free) culture medium, to which human tenascin-C was added such that its final concentration was 2 μg / mL, was added, and a normal rabbit IgG, the 4F10TT antibody or the anti-human TNIIIA2 a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
| concentrations | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com