Neurotherapeutic Cephalosporin Sulfoxide and Sulfone-Containing Compositions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example i
Synthesis of Δ2- and Δ3-Cefazolin Sulfoxides, Sodium Salts
[0059]Cefazolin. A solution of cefazolin, sodium salt, (998 mg, 2.095 mmol, commercial available from Chemifarma S.A., Madrid, Spain; Fujian Fukang Pharmaceutical Co., Ltd., Fuzhou, China) in water (100 mL) was treated with 1N aq. HCl (to pH 2.90). The resulting precipitate was suction-filtered and washed with water. The filtrate was made alkaline by treatment with 1N aq. NaOH (to pH 3.50), and then extracted with ethyl acetate (2×20 mL). The ethyl acetate extract was dried over Na2SO4, filtered, evaporated, and combined with the filtered material and dried to give 751 mg (79%) of the title compound.
[0060]Δ2- and Δ3-Cefazolin diphenylmethyl esters. To generate diphenyldiazomethane, Pb(OAc)4 (986 mg, 2.22 mmol) was added to a stirred solution of benzophenone hydrazone (437 mg, 2.22 mmol) in dichloromethane (40 mL) containing tetramethylguanidine (3.84 g, 33.4 mmol) at −78° C. under N2. After seventy min the reaction was quench...
example ii
Anxiety Behavioral Study: Dose-Response in the Seed Finding Model of Anxiety
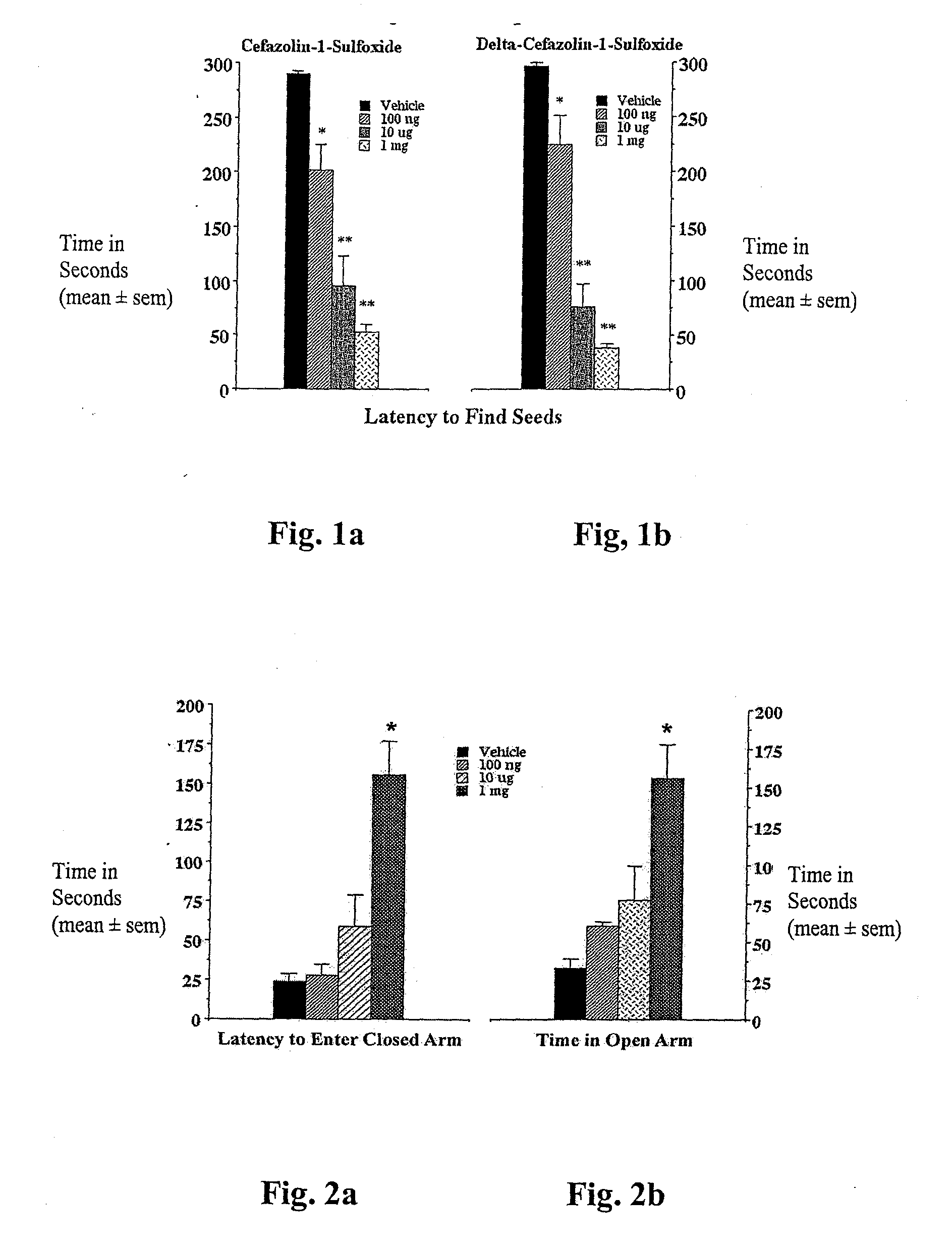
[0066]Rationale. The golden hamster seed finding model of anxiety is a robust and simple bioassay for screening beta-lactams for CNS activity. Briefly, hamsters are deprived of food overnight. The following day they are exposed to the additional stress of being taken from their home cage and placed in a novel environment for a few minutes. During their absence from the home cage, sunflower seeds are hidden under the bedding in one of the corners. When returned to the home cage, hamsters routinely scramble along the walls for 1-2 minutes before settling down, locating and eating the seeds. However, animals treated with the traditional anxiolytics, e.g., chlordiazepoxide, fluoxetine, or buspirone find seeds in less than 20 seconds (King J A, et al. (2001) Neuropsychobiology 45:150-155). This reduction in seed finding time from minutes to seconds also occurs following treatment with certain beta-lactam antibiot...
example iii
Anxiety Behavioral Study: Anxiolytic Activity in the Elevated Plus-Maze
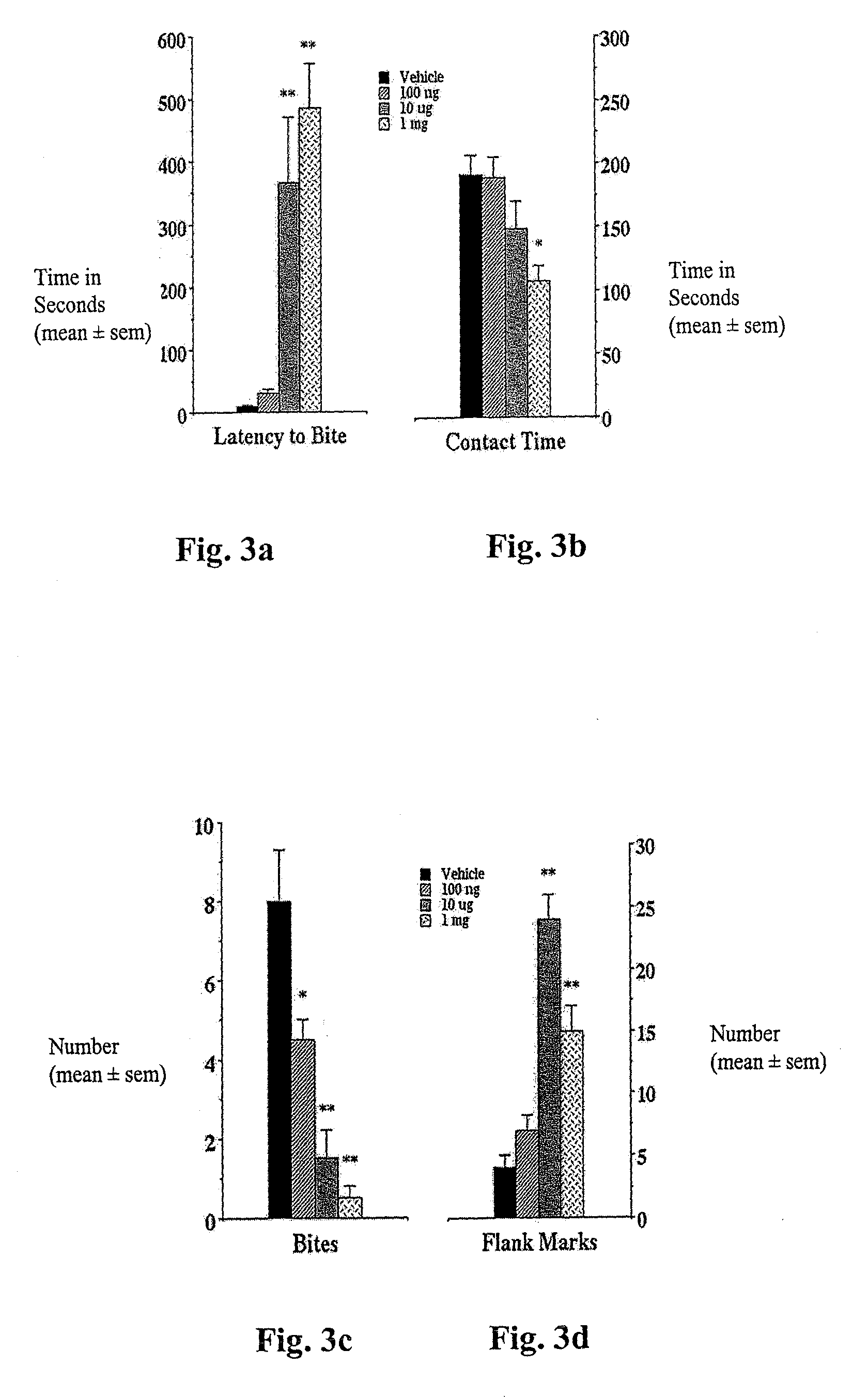
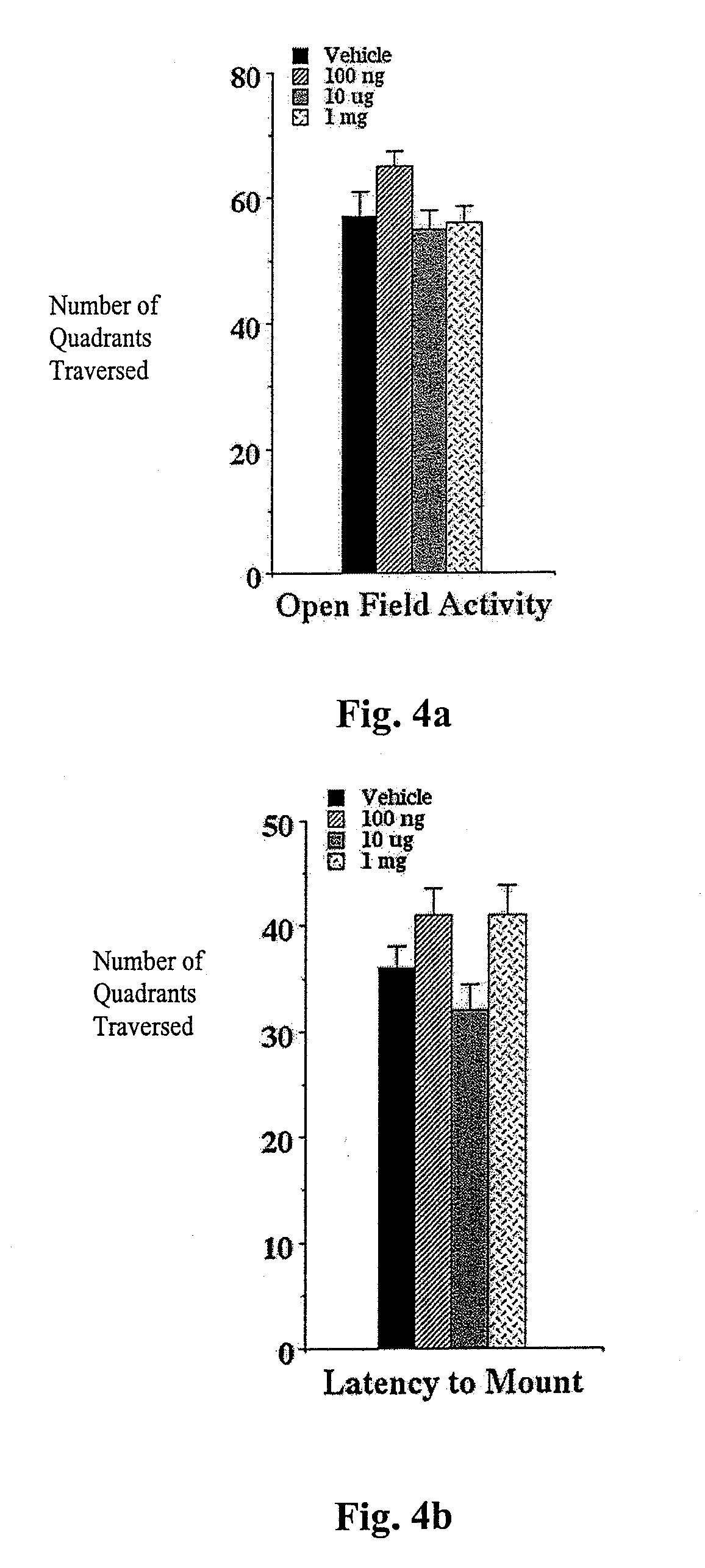
[0070]The elevated plus-maze was developed for the assessment of anxiolytic and anxiogenic drug effects in the rat (Pellow et al., (1985) Journal of Neuroscience Methods 14:149-167). The method has been validated behaviorally, physiologically, and pharmacologically. The plus-maze has two open arms and two enclosed arms. Rats will naturally make fewer entries into the open arms (light) than into the closed arms (dark) and will spend significantly less time in open arms. Confinement to the open arms is associated with significantly more anxiety-related behavior and higher stress hormone levels than confinement to the closed arms. Clinically effective anxiolytics, e.g., chlordiazepoxide or diazepam, significantly increase the percentage of time spent in the open arms and the number of entries into the open arms. Conversely, anxiogenic compounds such as yohimbin or amphetamines reduce open arm entries and time spent ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| suction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com