Injectable Melphalan Compositions Comprising a Cyclodextrin Derivative and Methods of Making and Using the Same
a technology of cyclodextrin and composition, which is applied in the field of injectable melphalan compositions and cyclodextrin derivatives, can solve the problems of inability to be refrigerated, and achieve the effects of improving the rate of therapeutic onset, reducing toxicology and side effects, and improving bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0149]The dissolution rate of free-base melphalan (Chemwerth, Woodbridge, Conn.) in solutions at various pH and at various concentrations of a cyclodextrin derivative were examined. The procedure was as follows: free base melphalan was added to a solution containing a cyclodextrin derivative (SBE6.5-β-CD, CAPTISOL®) and then vortex mixed for 1-5 minutes, and, if necessary, sonicated in ice water until a clear solution was achieved.
TABLEDissolution times for free base melphalan as a function ofcyclodextrin derivative concentration, volume, and pH.TargetDissolutionMelphalanSBE6.5-β-CDTimeConc.Conc.VolumepH(min)50 mg / mL200mM5 mL55050 mg / mL125mM6 mL59050 mg / mL100mM7 mL516050 mg / mL75mM8 mL5>18050 mg / mL50mM10 mL 536050 mg / mL125mM6 mL2.77550 mg / mL125mM10 mL 1.81650 mg / mL125mM6 mL1.3550 mg / mL75, 100 125mM10 mL 1.1
[0150]Referring to the data in the above Table, the dissolution of free base melphalan was very rapid at pH 1.1 regardless of the concentration of the cyclodextrin derivative. Afte...
example 3
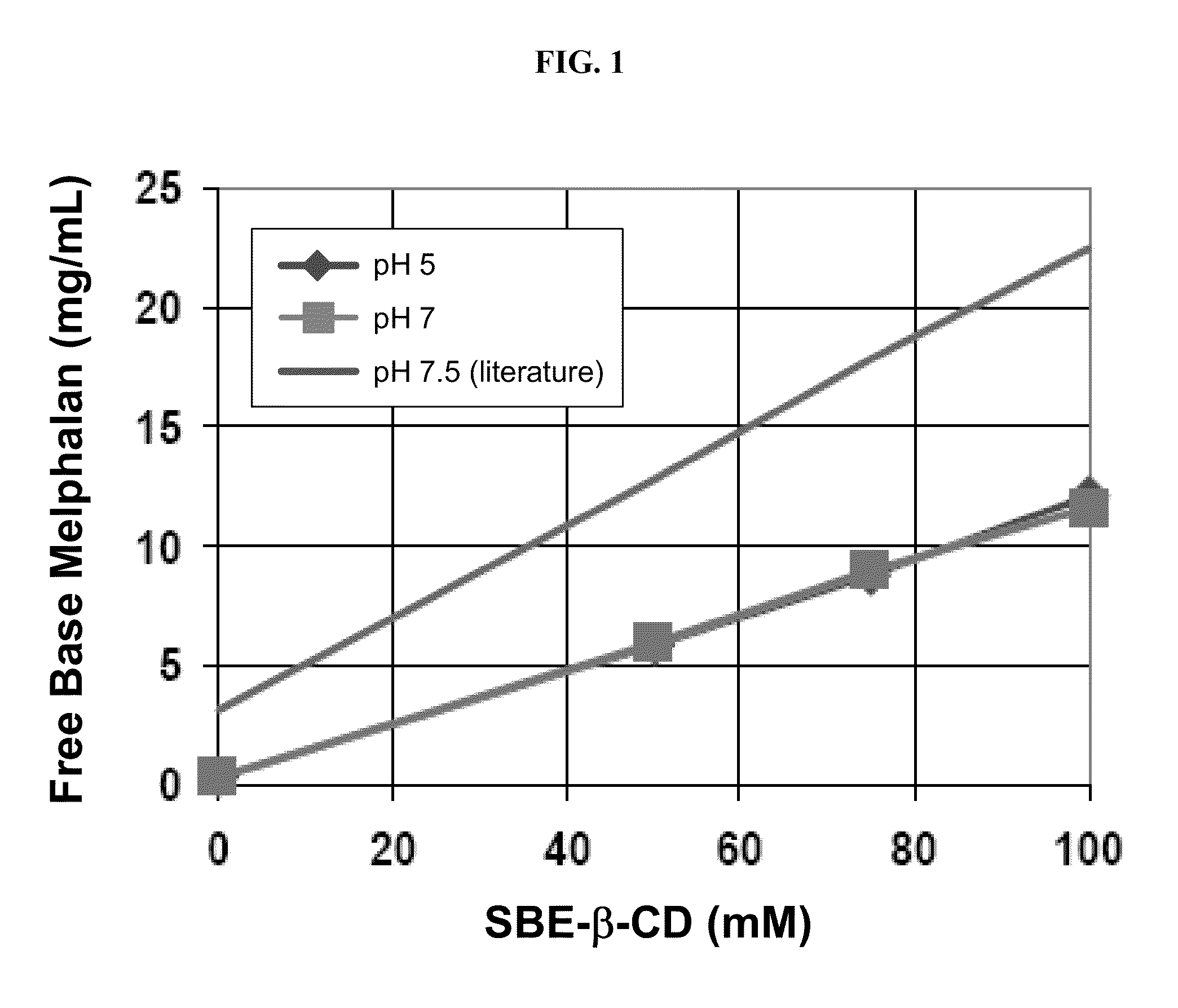
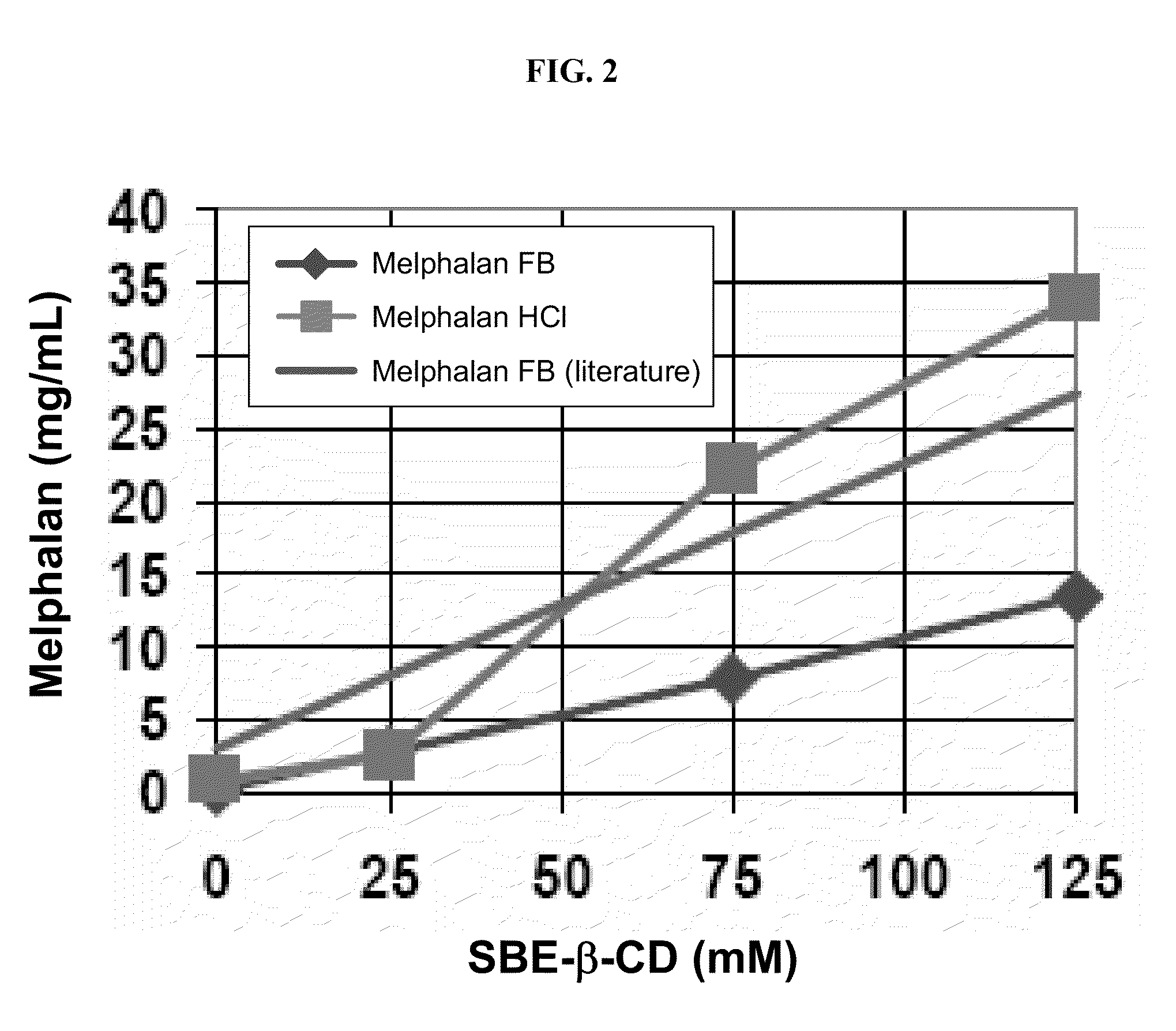
[0157]The binding of melphalan hydrochloride (USP reference standard) and free base melphalan (Chemwerth) with a cyclodextrin derivative (SBE6.5-β-CD, CAPTISOL®, avg. M.W.=2163 g / mol) was determined as a function of cyclodextrin derivative concentration at pH 7.5. The temperature was maintained at 22° C., and a 25 mM phosphate buffer was added to each solution. The data was compared with a literature report of free-base melphalan binding with SBE7-β-CD (avg. M.W.=2248 g / mol) in a 25 mM phosphate buffer at pH 7.5 (see Example 2).
[0158]The samples were prepared by adding excess melphalan hydrochloride or free base melphalan to a 1 mL sample of various SBE6.5-β-CD solutions. The samples were vortex mixed for 30 seconds, sonicated at 20-24° C. for 60 minutes, and then mixed by end-over-end rotation at 22° C. for 60 minutes. The samples were then centrifuged, the clear supernatant was diluted with water, and analyzed by HPLC. The data are provided in FIG. 2. Referring to FIG. 2, the melp...
example 4
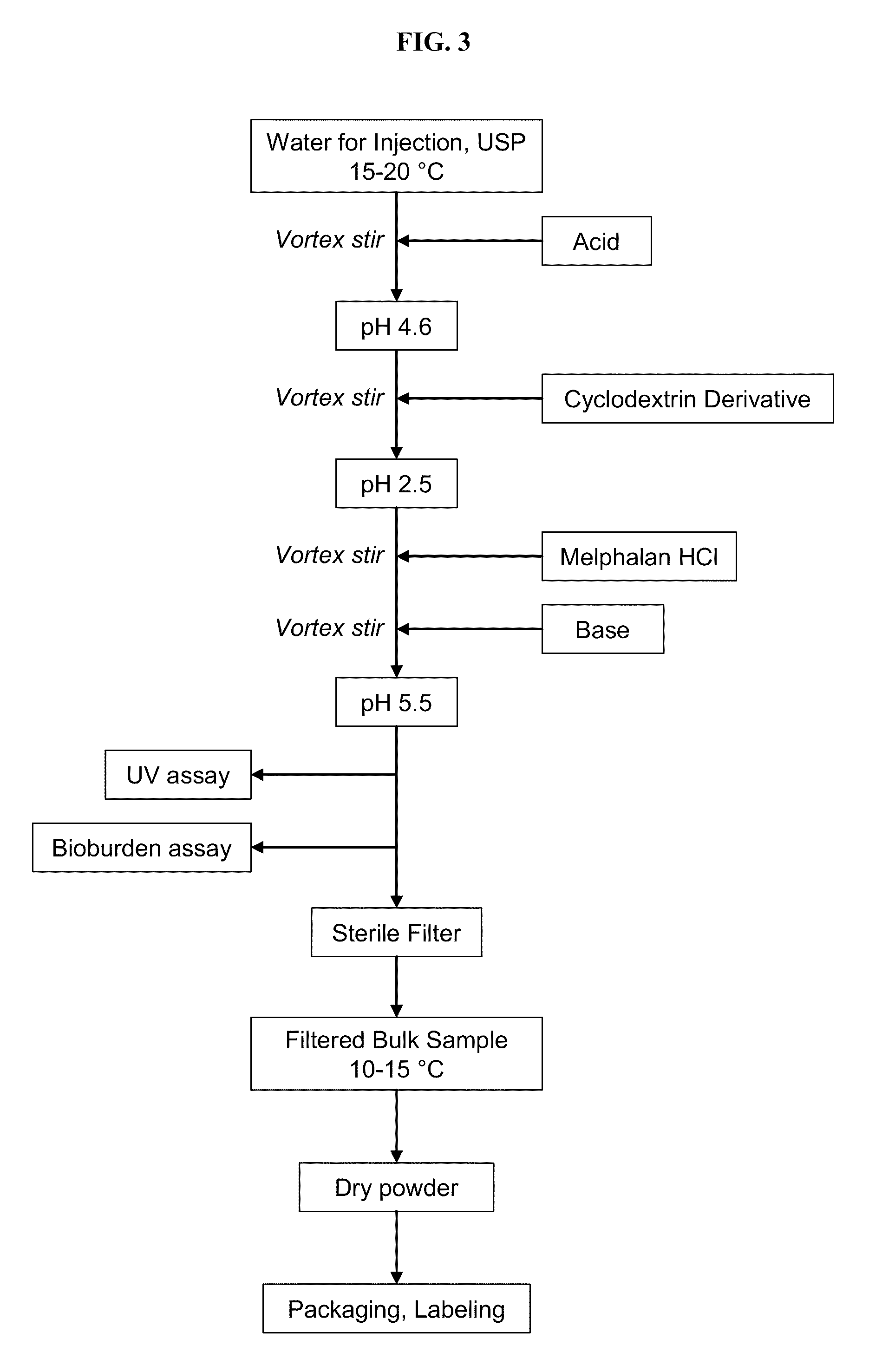
[0159]A pharmaceutical composition comprising melphalan as a hydrochloride salt was prepared by the process outlined schematically in FIG. 3. Referring to FIG. 3, water for injection, USP was placed in a stainless steel mixer at a temperature of 15-20° C., and hydrochloric acid was added until a pH of about 4.6 was achieved. The resulting solution was stirred at a speed sufficient to produce a vortex (but without foaming or frothing) for about 15 minutes, a cyclodextrin derivative (27.2 g SBE6.5-β-CD, CAPTISOL®) was added slowly while vortex stirring, and the resulting solution was stirred for about 15 minutes to ensure complete dissolution. The resulting solution had a pH of about 2.5. Melphalan as a hydrochloride salt (516 mg) was added slowly while vortex stirring, and the resulting solution was stirred for about 15 minutes to ensure complete dissolution. A base (2 N NaOH) was then slowly added while vortex stirring until the solution had a pH of about 5.6. The solution was then ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com