Method of using cytokine assay to diagnose, treat, and evaluate inflammatory and autoimmune diseases
a cytokine assay and cytokine technology, applied in the field of chronic inflammatory diseases, can solve the problems of establishing a definitive link between cytokine expression and diagnosis, prognosis, and prescription of these expensive, powerful and potentially dangerous therapeutic agents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Distinct Cytokine Patterns in Ankylosing Spondylitis (AS)
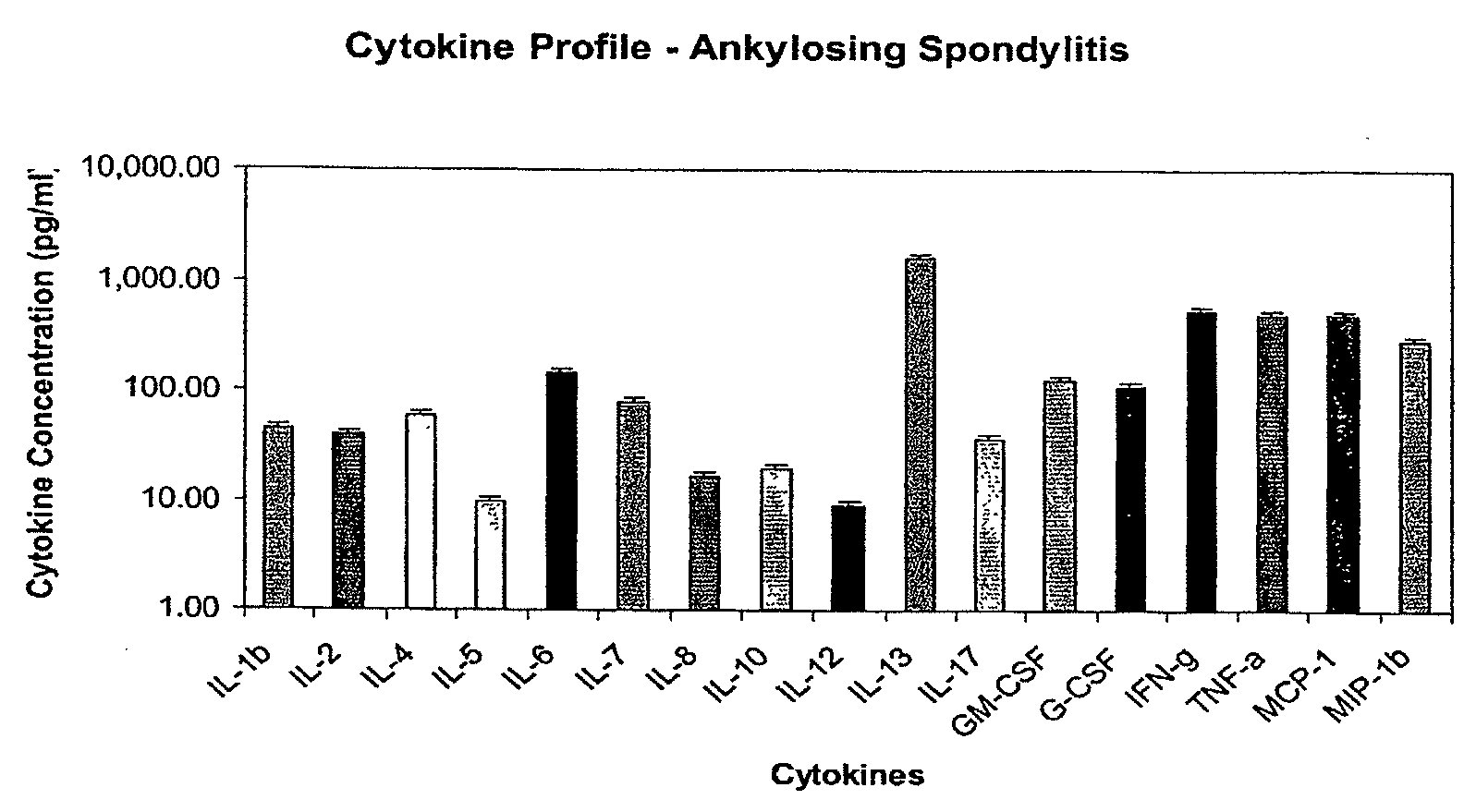
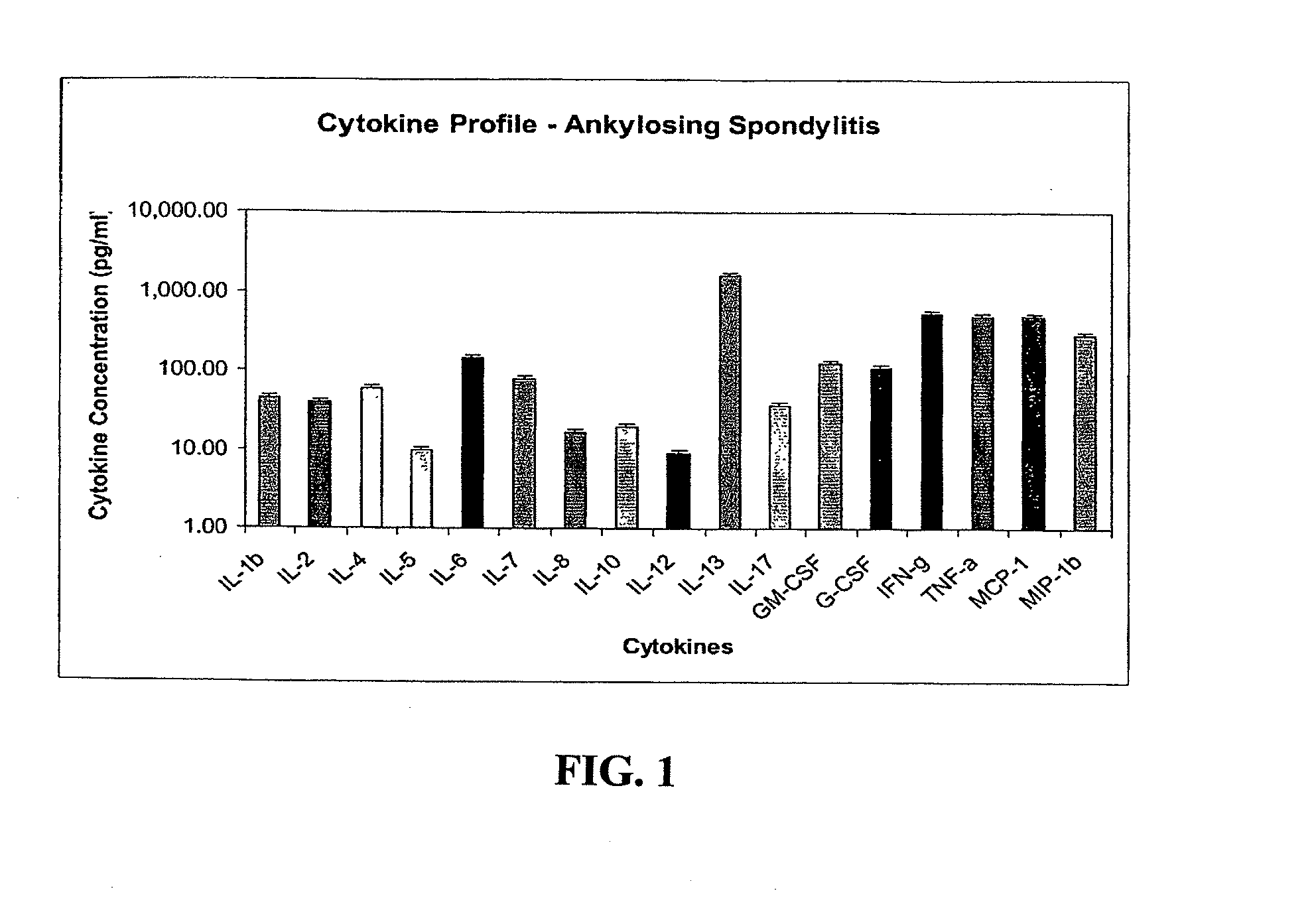
[0461]A distinct profile of cytokines was generated from patients with ankylosing spondylitis (AS). This cytokine profile was determined by sampling peripheral blood serum for the presence of cytokines. Patients were found to have predictive molecular cytokine profiles based on clinical disease phenotype and disease severity. Specifically, cytokines were found to be similar within the specific disease classifications, but the levels of cytokines were somewhat heterogeneous with regard to individual patient, raising the possibility that various stages of disease and disease severity may be distinguished by this molecular diagnostic mechanism.
[0462]1. Study Population
[0463]Peripheral blood serum was obtained from more than 44 patients with active ankylosing spondylitis who fulfilled the diagnostic criteria and classification by the European Spondylarthropathy Study Group (ESSG)—Bath Criteria (Calin, A., J. D. Taurog., Eds. 1998,...
example 2
Distinct Cytokine Patterns in Psoriatic Arthritis (PsA)
[0476]A distinct profile of cytokines was geneated from patients with psoriatic arthritis (PsA). This distinct cytokine profile was determined by sampling serum and cerebrospinal fluid for the presence of cytokines. Patients were found to have predictive molecular cytoline profiles based on clinical disease phenotype and disease severity. Specifically, cytokines were found to be similar within the specific disease classifications, but the levels of cytokines were somewhat heterogeneous with regard to individual patient, raising the possibility that various stages of disease and disease severity may be distinguished by this molecular diagnostic mechanism.
[0477]1. Study Population
[0478]Peripheral blood serum from seventy-two (72) patients diagnosed with psoriatic arthritis were analyzed. Cerebrospinal fluid from one patient was also analyzed.
[0479]2. Cytokine Measurement
[0480]The samples were analyzed using a comprehensive biometr...
example 3
Distinct Cytokine Patterns in Reactive Arthritis (ReA)
[0487]A distinct profile of cytokines was produced from patients with reactive arthritis (ReA). This distinct cytokine profile was determined by sampling serum for the presence of cytokies. Patients were found to have predictive molecular cytokine profiles based on clinical disease phenotype and disease severity. Specifically, cytokines were found to be similar within the specific disease classifications, but the levels of cytokines were somewhat heterogeneous with regard to individual patient, raising the possibility that various stages of disease and disease severity may be distinguished by this molecular diagnostic mechanism.
[0488]1. Study Population
[0489]Peripheral blood serum from 13 patients diagnosed with reactive arthritis were analyzed.
[0490]2. Cytokine Measurement
[0491]The samples were analyzed using a comprehensive biometric multiplex cytoline assay (BioSource, Camarillo, Calif., USA) (described above).
[0492]3. Statist...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| pH | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com