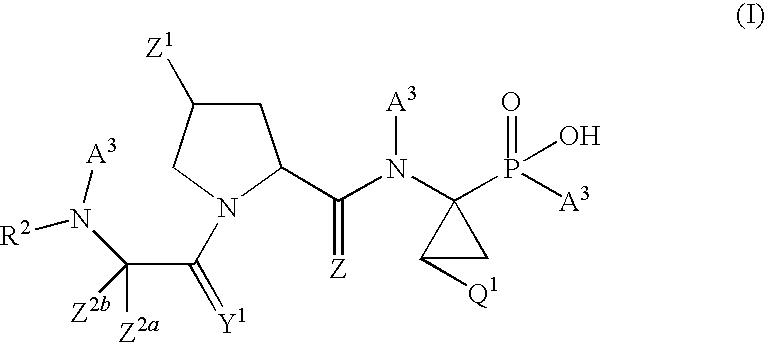
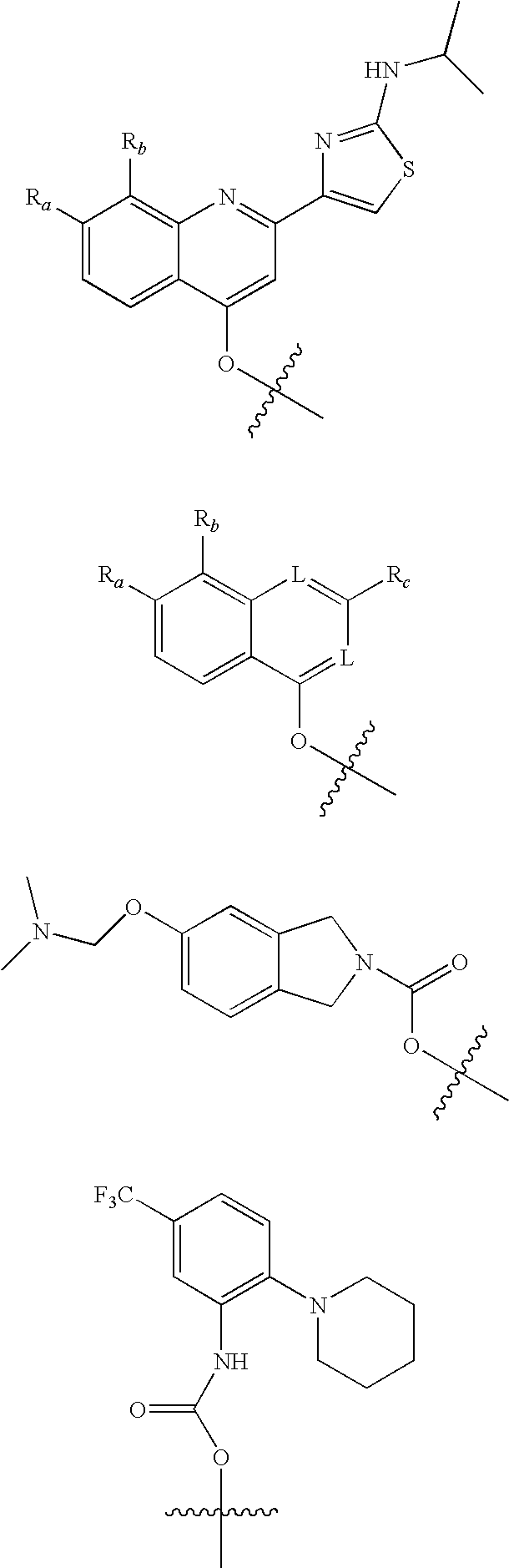
Antiviral phosphinate compounds
a technology of phosphinate and compound, which is applied in the direction of peptides, drug compositions, peptides, etc., can solve the problems of limited usefulness of effects, and achieve the effects of improving oral bioavailability, enhancing activity against development, and improving inhibitory or pharmacokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of Compound 1
[0383]
[0384]Phosphonic acid intermediate (1-benzyloxycarbonylamino-2-vinyl-cyclopropyl)-phosphonic acid monoethyl ester III (415 mg, 1.28 mmol) was dissolved in toluene (8 mL). This solution was cooled to 0° C. and (COCl)2 (222 μL, 2.56 mmol) was added in a drop-wise fashion. DMF (44 μL, 0.56 mmol) was then added. The reaction was run for 2 h at 0° C. and determined to be complete by 31P NMR. 31P NMR (121.4 MHz, CDCl3): δ 39.0, 38.5, 37.4, 36.5, 17.0, 16.2, 16.0, 15.4.
[0385]The reaction was concentrated to orange-yellow oil and then placed under high vacuum for 1 h. The resulting residue was dissolved in THF (6.4 mL) and this solution was cooled to −78° C. A 1.4 M solution of methyllithium in diethyl ether (1.37 mL, 1.92 mmol) was added drop-wise. After 40 min, more methyllithium (456 μL, 0.64 mmol) was added drop-wise. After 10 min, the reaction was quenched at −78° C. by the addition of sat. NH4Cl(aq.). The organic phase was diluted with EtOAc and extracte...
example 2
Preparation of Compound 2
[0389]
[0390]Phosphonic acid intermediate III (208 mg, 0.64 mmol) was dissolved in toluene (8 mL). This solution was cooled to 0° C. and (COCl)2 (111 μL, 1.28 mmol) was added in a drop-wise fashion. DMF (22 μL, 0.28 mmol) was then added. The reaction was run for 2 h at 0° C. and determined to be complete by 31P NMR. 31P NMR (121.4 MHz, CDCl3): δ 39.0, 38.5, 37.4, 36.5, 17.0, 16.2, 16.0, 15.4.
[0391]The reaction was concentrated to orange-yellow oil and then placed under high vacuum for 1 h. The resulting residue was dissolved in THF (6.4 mL) and this solution was cooled to −78° C.
[0392]A solution of EtLi in dibutyl ether (1.7 M, 566 μL, 0.96 mmol) was added drop-wise. After 40 min, more EtLi (189 μL, 0.32 mmol) was added drop-wise. After 10 min, the reaction was quenched at −78° C. by the addition of sat. NH4Cl(aq.). The organic phase was diluted with EtOAc and extracted with sat. NH4Cl(aq.) and brine. The organic phase was dried over MgSO4. Concentration of t...
example 3
Preparation of Compound 3
[0395]
[0396]Phosphonic acid intermediate III (386 mg, 1.19 mmol) was dissolved in toluene (14.9 mL). This solution was cooled to 0° C. and (COCl)2 (155 μL, 1.78 mmol) was added in a drop-wise fashion. DMF (20 μL, 0.26 mmol) was then added. The reaction was run for 2 h at 0° C. and determined to be complete by 31P NMR. 31P NMR (121.4 MHz, CDCl3): δ 39.0, 38.5, 37.4, 36.6, 17.0, 16.2, 16.1, 15.4.
[0397]The reaction was concentrated to a yellow-orange oil and then placed under high vacuum for 1 h. The resulting residue was dissolved in THF (11.9 mL) and this solution was cooled to −78° C. A 2.0 M solution of n-BuLi in pentane (595 μL, 1.19 mmol) was added drop-wise. After 40 min more n-BuLi (520 μL, 1.04 mmol) was added drop-wise. After 10 min the reaction was quenched at −78° C. by the addition of sat. NH4Cl(aq.). The organic phase was diluted with EtOAc and extracted with sat. NH4Cl(aq.) and brine. The organic phase was dried over MgSO4. Concentration of the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com