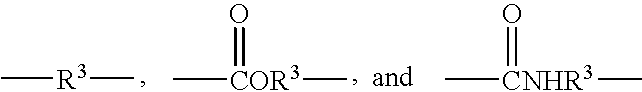Curable compositions for optical articles
a technology of compositions and optical articles, applied in the field of curable compositions, can solve the problems of optical defects, unpredictability of registration, net shrinkage in volume, etc., and achieve the effects of low birefringence, low sensitivity to moisture, and low shrinkag
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
examples
[0119] These examples are merely for illustrative purposes only and are not meant to be limiting on the scope of the appended claims. All parts, percentages, ratios, etc. in the examples and the rest of the specification are by weight, unless noted otherwise. Solvents and other reagents used were obtained from Sigma-Aldrich Chemical Company; Milwaukee, Wis. unless otherwise noted.
Table of AbbreviationsAbbreviation orTradeDesignationDescriptionIBOAIsobornyl acrylate, available from SartomerCompany Inc, Exton, PAMMAMethyl methacrylateHEAHydroxyethyl acrylateHEMAHydroxyethyl methacrylateIOTGIsooctyl thioglycolate, available from TCIAmerica, Portland, ORMCEMercaptoethanolIEMIsocyanatoethyl methacrylate, available from ShowaDenka, JapanMAnhMethacrylic anhydrideHDDMA1,6 Hexanediol dimethacrylate, SR239, availablefrom Sartomer Company Inc, Exton, PAVazo 522,2′-Azobis(2,4-dimethylvaleronitrile), availablefrom DuPont Company, Wilmington, DEVazo 881,1′-Azobis(cyanocyclohexane), available fr...
examples 1-7
Preparation of Oligomer Syrups:
[0126] In Examples 1-7, IBOA, HEA, chain transfer agent IOTG or MCE, and the 1st charge of thermal initiators Vazo 52 and 88, according to Table 1, were added to a four neck flask equipped with a reflux condenser, thermometer, mechanical stirrer, and nitrogen gas inlet. The mixture was stirred and heated to 60° C. under nitrogen. The temperature of the reaction mixture peaked at around 150° C. during the polymerization. After the reaction peak, the batch was further polymerized at 140° C. for 30 minutes with the addition of the 2nd initiator, Vazo 88, to reduce residual monomers and eliminate initiator. A sample was taken at the end of this reaction period for oligomer molecular weight determination by SEC. After that, the batch was cooled to 100° C. The HDDMA reactive diluent was added to the reactor to reduce viscosity of the batch. A solution of the DBDL catalyst in IEM was then added to the batch to react with the hydroxyls on the IBOA / HEA polyme...
examples 10-11
[0129] To prepare reactive oligomer syrups of Example 10 and 11 in Table 3, the same polymerization procedure described in the Examples 1-7 above was followed, except 30 minutes after the addition of the 2nd initiator at 140° C., MAnh was added and the mixture was reacted at 140° C. for another 3 hours with efficient stirring. After that, the batch was cooled down to 110° C. and HDDMA reactive diluent was added to the reactor to further reduce the viscosity of the batch.
TABLE 3Example1011IBOA (g)190160MMA (g)026HEA (g)1010HEMA (g)04IOTG (g)48Vazo 52 / (g)0.025 / 0.025 / Vazo 88 (g)0.0250.025Vazo 88 (g)0.0500.050MAnh (g)14.1219.16HDDMA (g)5035.3
PUM
| Property | Measurement | Unit |
|---|---|---|
| Temperature | aaaaa | aaaaa |
| Temperature | aaaaa | aaaaa |
| Fraction | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com