Antimicrobial amino acid sequences derived from alpha-melanocyte-stimulating hormone
a technology of alpha-melanocyte-stimulating hormone and amino acid sequences, which is applied in the direction of biocide, drug compositions, peptide/protein ingredients, etc., can solve the problems of pain and slow search for antimicrobial peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
aterials and Methods
Peptides
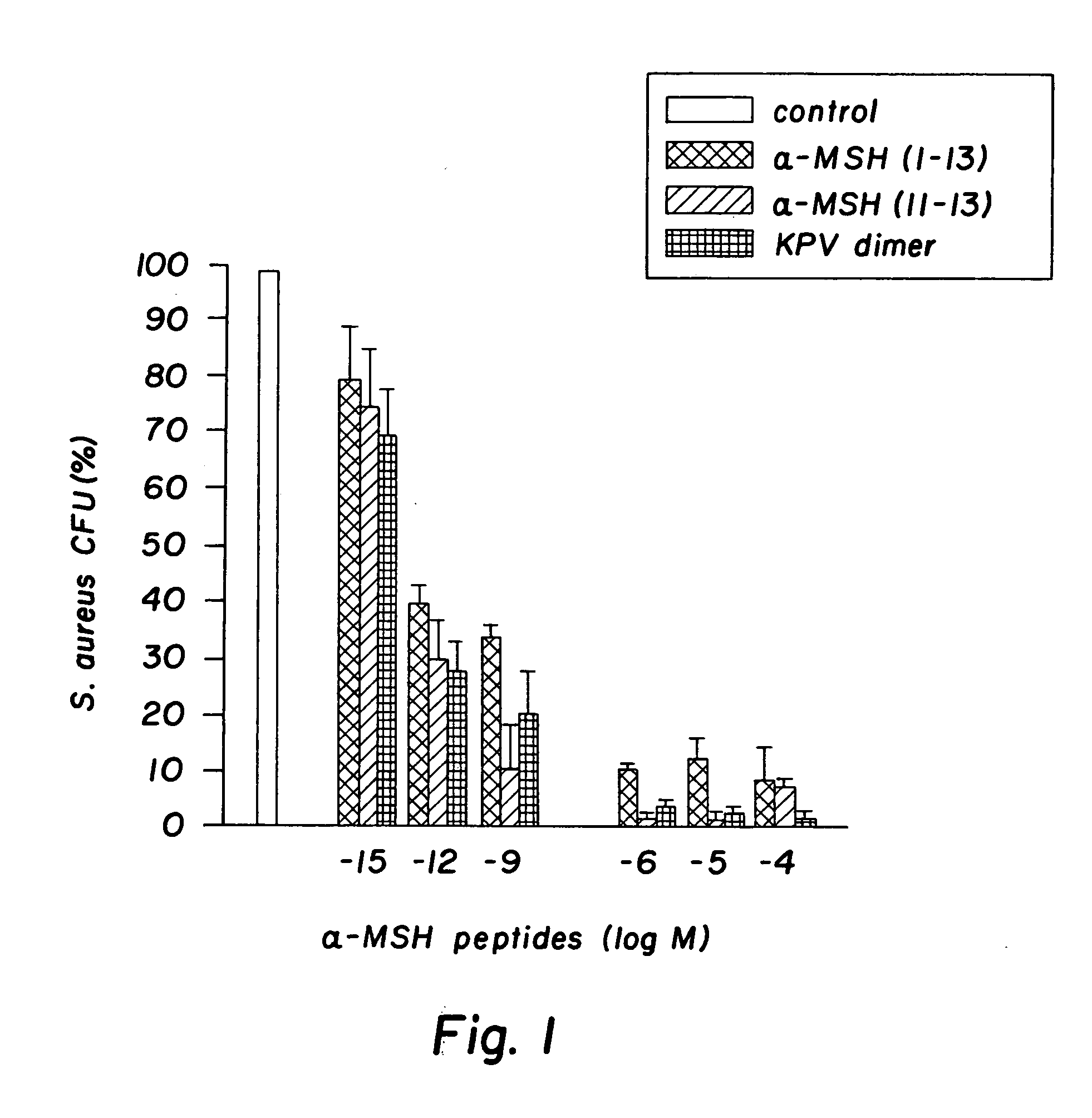
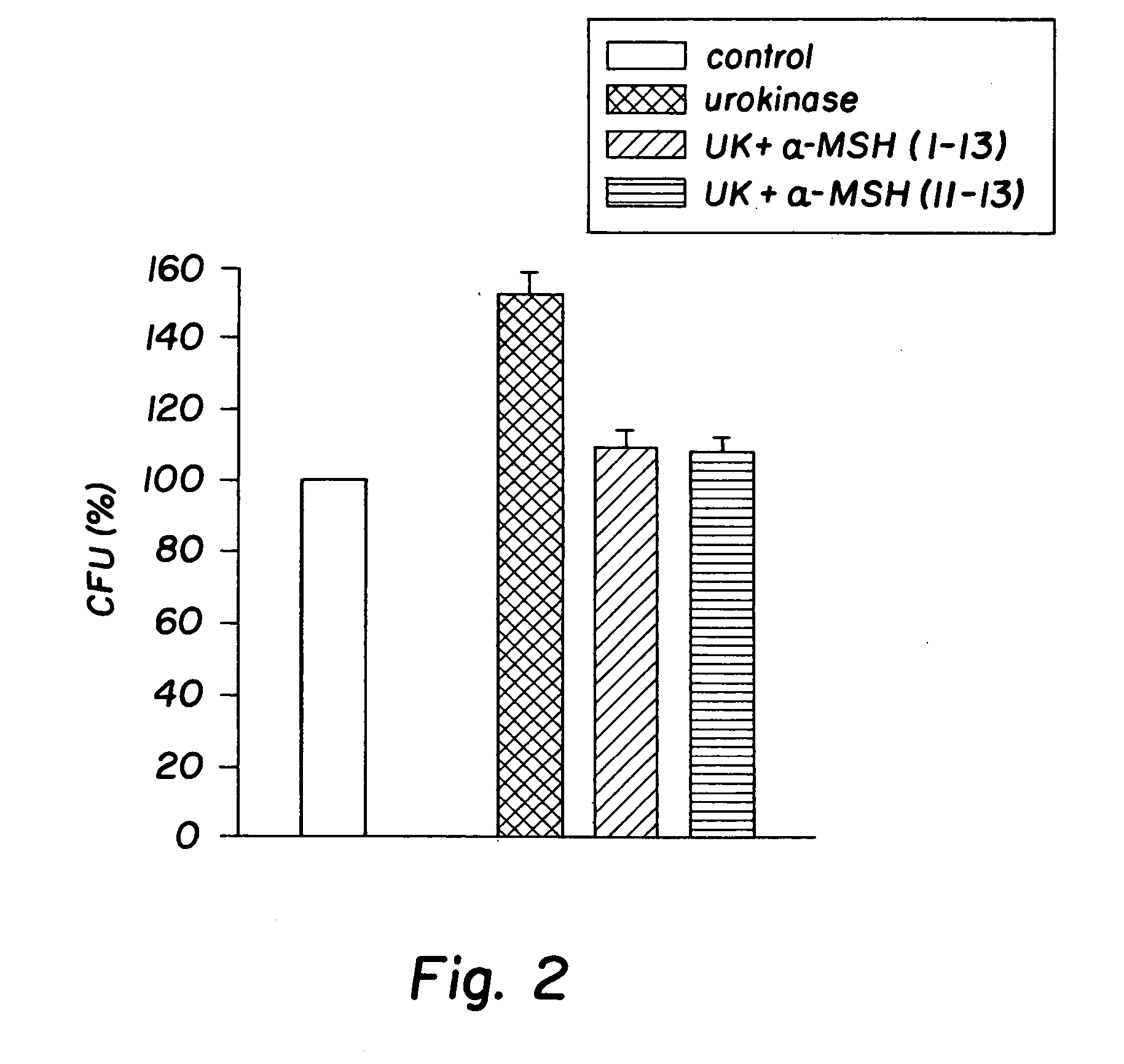
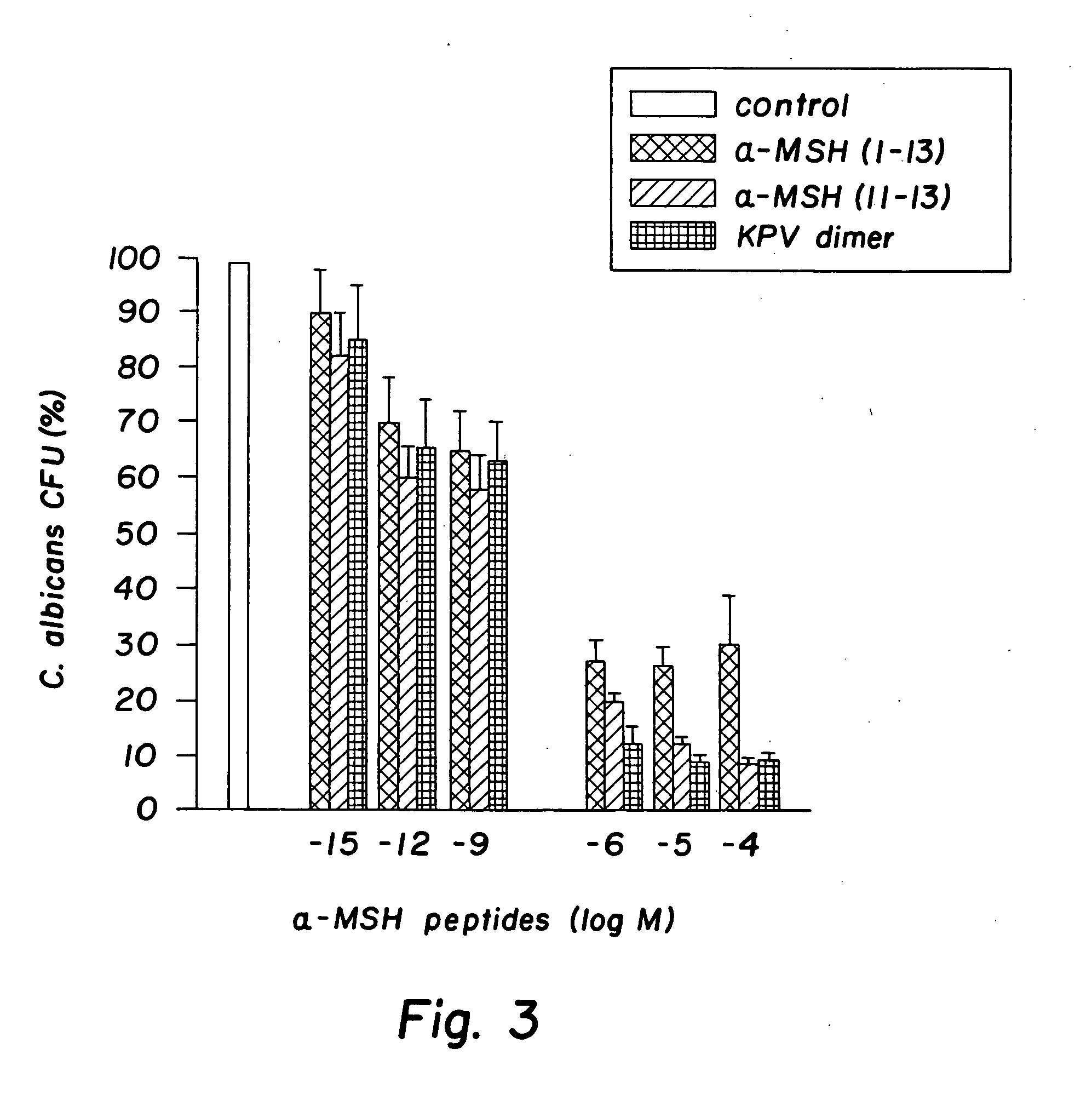
[0032] The peptides used in this research included: α-MSH (1-13), (4-10), (6-13), and (11-13), all of which were N-acetylated and C-amidated, and ACTH (1-39) and (18-39) (CLIP). Another peptide used in this research included a dimer of the amino acid sequence KPV, specifically VPKCCKPV, which also was N-acetylated and C-amidated (the “KPV dimer”). The KPV dimer can be chemically represented as NH2-Lys-Pro-Val-AcCys-CysAc-Val-Pro-Lys-NH2. The peptides were prepared by solid-phase peptide synthesis and purified by reversed-phase high performance liquid chromatography, as kindly provided by Dr. Renato Longhi, CNR, Milano.
Organism and Culture Conditions
[0033]S. aureus (ATCC 29213) and C. albicans (clinical isolate) were obtained from the collection of the Department of Microbiology, Ospedale Maggiore di Milano. C. albicans were maintained on Sabouraud's agar slants and periodically transferred to Sabouraud's agar plates and incubated for 48 hours at 28° ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| amino acid chain length | aaaaa | aaaaa |
| chain length | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com