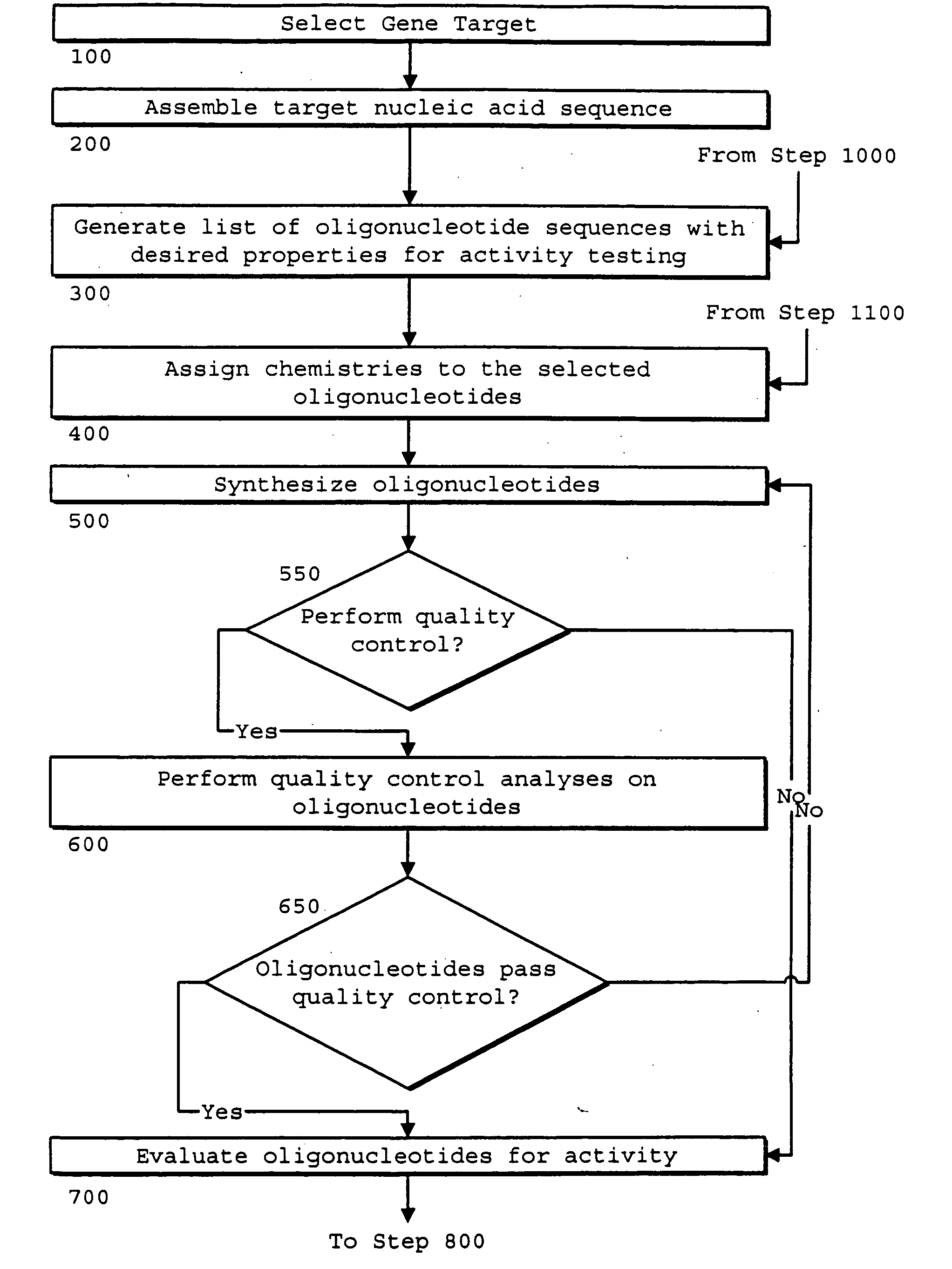
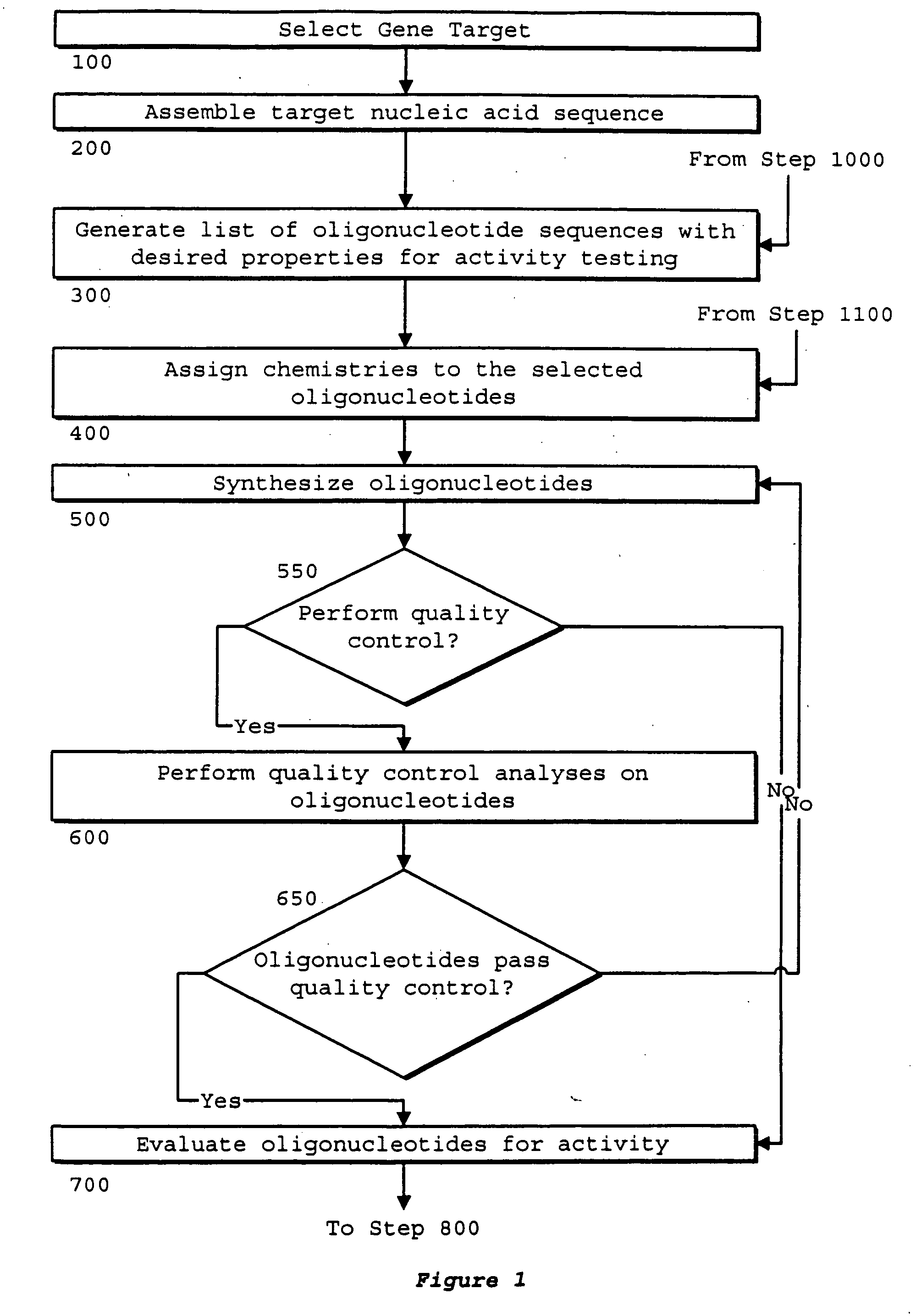
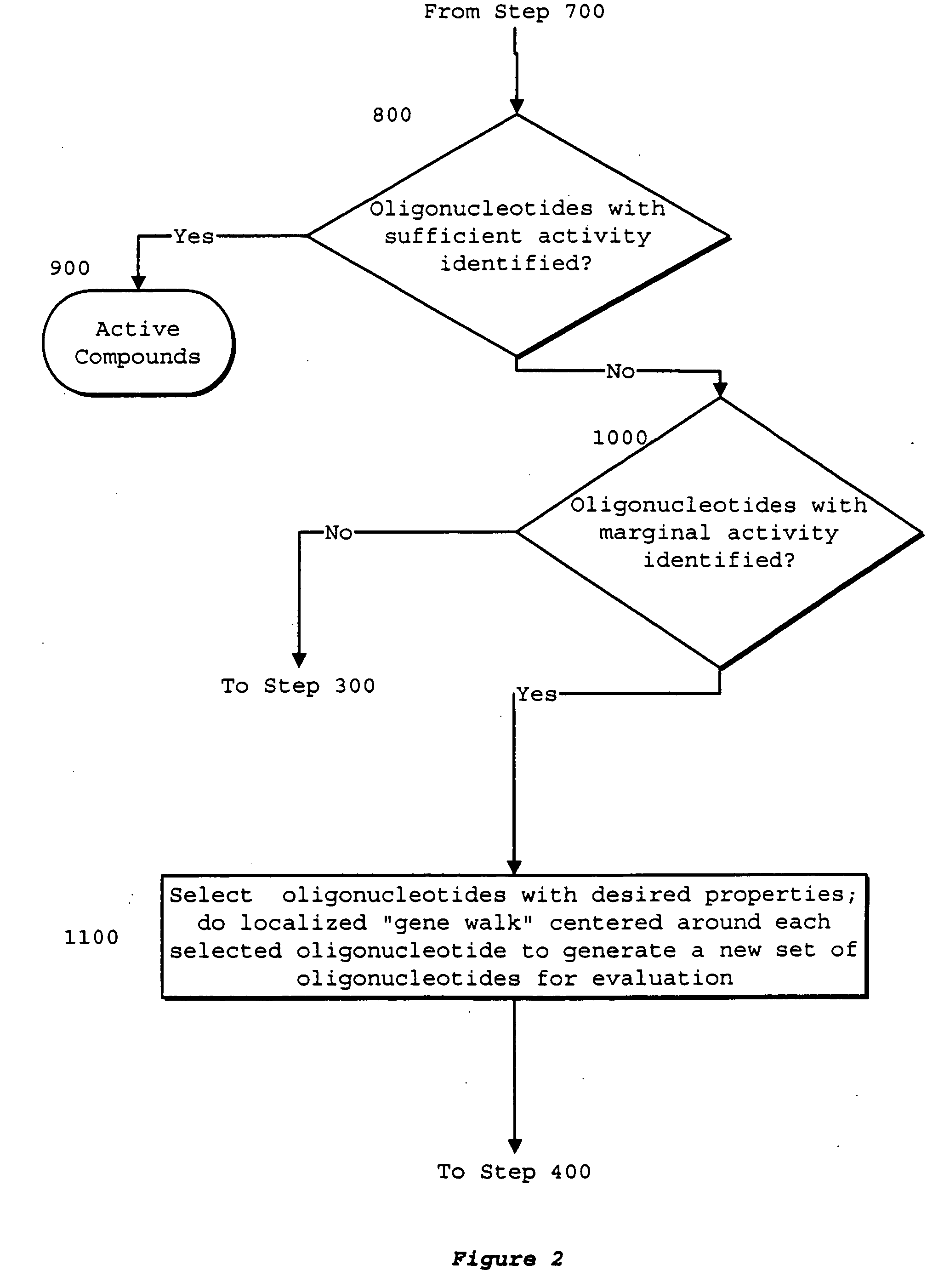
Identification of genetic targets for modulation by oligonucleotides and generation of oligonucleotides for gene modulation
a technology of gene modulation and gene target, applied in the field of synthetic compounds, can solve the problems of limited use of manual synthesis and analysis limited use of lead antisense compounds in the search for lead antisense compounds, and the conventional approach is dependent on the availability, number and cost of antisense compounds produced by manual or at best semi-automatic means
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Selection of CD40 as a Target
[0197] Cell-cell interactions are a feature of a variety of biological processes. In the activation of the immune response, for example, one of the earliest detectable events in a normal inflammatory response is adhesion of leukocytes to the vascular endothelium, followed by migration of leukocytes out of the vasculature to the site of infection or injury. The adhesion of leukocytes to vascular endotheliurn is an obligate step in their migration out of the vasculature (for a review, see Albelda et al., FASEB J., 1994, 8, 504). As is well known in the art, cell-cell interactions are also critical for propagation of both B-lymphocytes and T-lymphocytes resulting in enhanced humoral and cellular immune responses, respectively (for a reviews, see Makgoba et al., Immunol. Today, 1989, 10, 417; Janeway, Sci. Amer., 1993, 269, 72).
[0198] CD40 was first characterized as a receptor expressed on B-lymphocytes. It was later found that engagement of B-cell CD40 wi...
example 2
Generation of Virtual Oligonucleotides Targeted to CD40
[0202] The process of the invention was used to select oligonucleotides targeted to CD40, generating the list of oligonucleotide sequences with desired properties as shown in FIG. 22. From the assembled CD40 sequence, the process began with determining the desired oligonucleotide length to be eighteen nucleotides, as represented in step 2500. All possible oligonucleotides of this length were generated by Oligo 5.0™, as represented in step 2504. Desired thermodynamic properties were selected in step 2508. The single parameter used was oligonucleotides of melting temperature less than or equal to 40° C. were discarded. In step 2512, oligonucleotide melting temperatures were calculated by Oligo 5.0™.
[0203] Oligonucleotide sequences possessing an undesirable score were discarded. It is believed that oligonucleotides with melting temperatures near or below physiological and cell culture temperatures will bind poorly to target seque...
example 3
Input Files For Automated Oligonucleotide Synthesis Command File (.cmd File)
[0204] Table 2 is a command file for synthesis of oligonucleotide having regions of 2′-O-(methoxyethyl) nucleosides and region of 2′-deoxy nucleosides each linked by phosphorothioate internucleotide linkages.
TABLE 2SOLID_SUPPORT_SKIPBEGINNext_SequenceENDINITIAL-WASHBEGINAdd ACN 300Drain 10ENDLOOP-BEGINDEBLOCKBEGINPrime TCALoad TrayRepeat 2Add TCA 150Wait 10Drain 8End_RepeatRemove TrayAdd TCA 125Wait 10Drain 8ENDWASH_AFTER_DEBLOCKBEGINRepeat 3Add ACN 250 To_AllDrain 10End_RepeatENDCOUPLINGBEGINif class = DEOXY_THIOATENozzle wash prime prime Add 70 + 70Wait 40Drain 5end-ifif class = MOE_THIOATENozzle wash Prime prime Add 120 + 120Wait 230Drain 5End_ifENDWASH_AFTER_COUPLINGBEGINAdd ACN 200 To_AllDrain 10ENDOXIDIZEBEGINif class = DEOXY_THIOATEAdd BEAU 180Wait 40Drain 7end_ifif class = MOE_THIOATEAdd BEAU 200Wait 120Drain 7end_ifENDCAPBEGINAdd CAP_B 80 + CAP_A 80Wait 20Drain 7ENDWASH_AFTER_CAPBEGINAdd ACN ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| volume | aaaaa | aaaaa |
| internal pressure | aaaaa | aaaaa |
| volume | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com