Novel immobilized calixarenes and related compounds and process for their production
a technology of immobilized calixarene and calixarene, which is applied in the direction of physical/chemical process catalysts, organic compound/hydride/coordination complex catalysts, other chemical processes, etc., can solve the problems of weak binding, requiring rather laborious calixarene synthesis, and poor solubility of calixarene in these solvents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
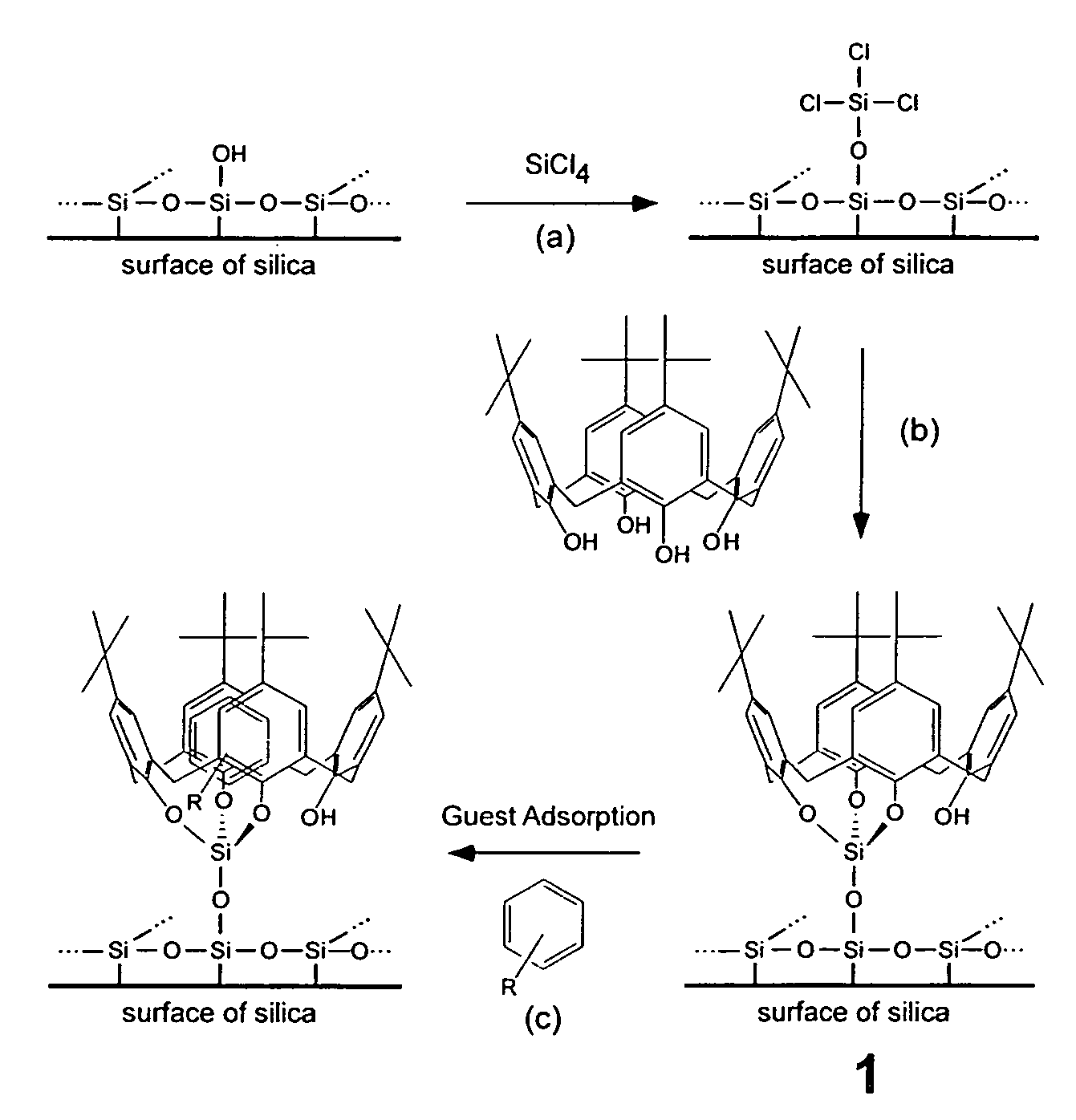
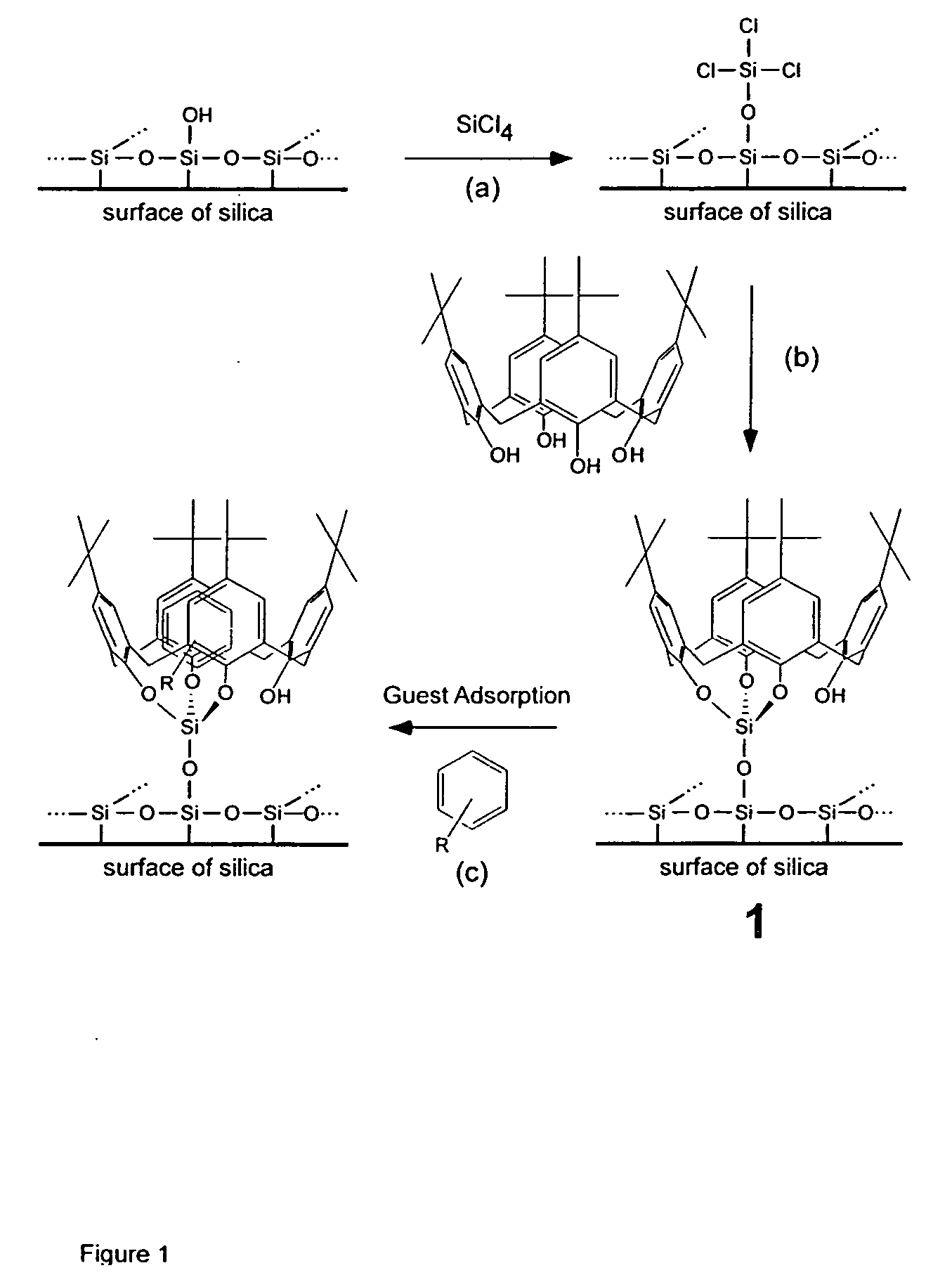
Immobilization of t-buytlcalix[4]arene Using Silica
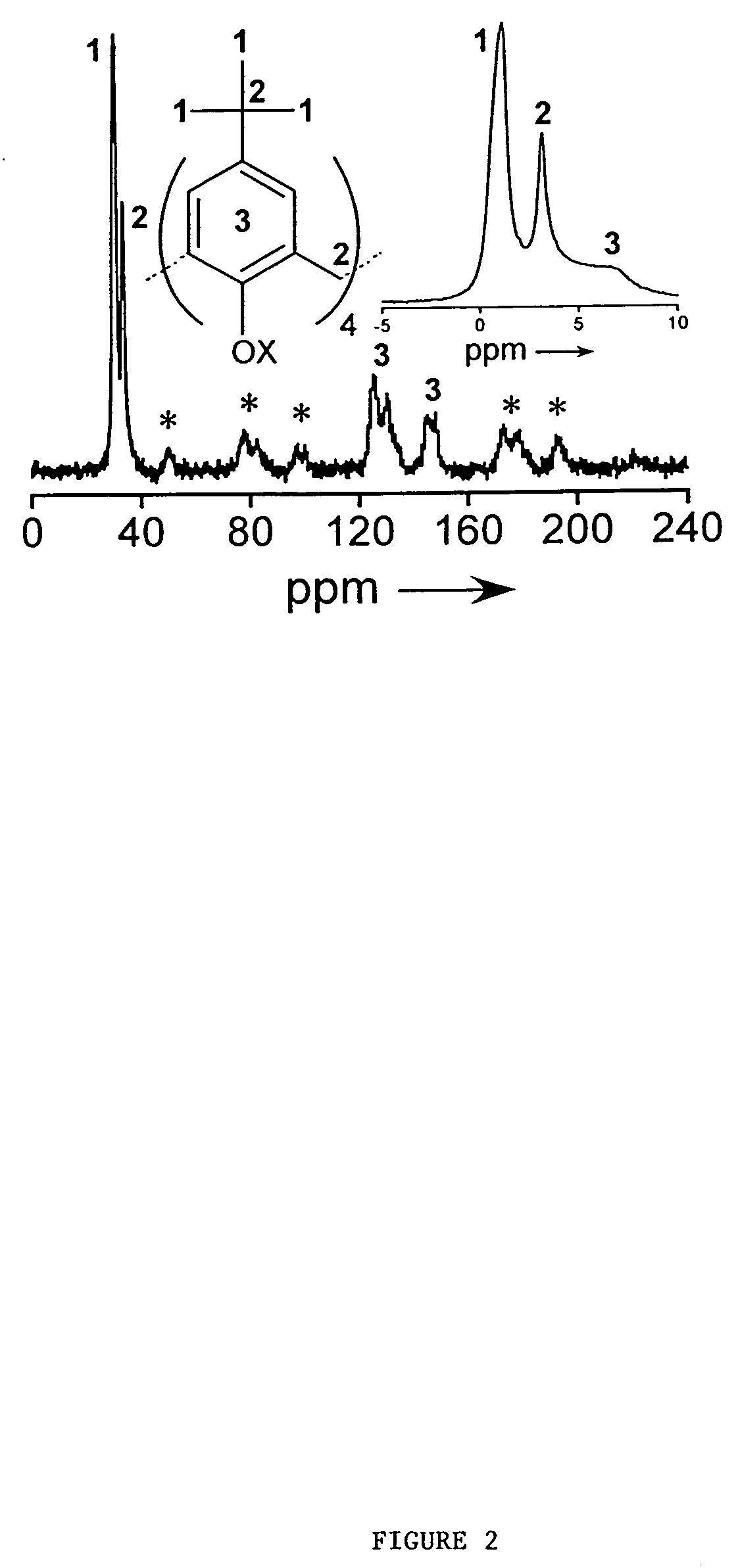
[0048] Synthesis: t-Butylcalix[4]arene immobilized on a silica substrate (1) was prepared starting with 1.5 g of silica, which was dehydrated at 300° C. under a vacuum of at least 50 mtorr for 24 hours in a Schlenk tube. After cooling under nitrogen to room temperature, a solution of SiCl4 in dichloromethane (1M, 6.8 mL) was added via dry syringe, followed by the addition of triethylamine (0.69 g, 6.8 mmol). The resulting cloudy solution was kept at room temperature for at least 12 h, and the solvent was subsequently evaporated in vacuo to yield a dry white powder. A solution of the macrocyclep-tert-butyl-calix-[4]-arene (0.3 g, 0.46 mmol) in toluene (20 mL) was freshly prepared, and the white powder was added by repeated rinsings of the Schlenk tube with toluene (30 mL). Finally triethylamine (1.75 g, 17.3 mmol) was added to this mixture, and the resulting solution was refluxed under nitrogen for a period of 24 h, with the condens...
example 2
Adsorption of NO Using Immobilized Calixarene
[0057] The gas-phase adsorption of NO to 1 was also investigated, since it is known that NO strongly adsorbs into the lipophilic cavity ofp-tert-butylcalix-[4]-arene via cofacial interaction with the aromatic groups. Experiments were conducted by first pretreating 1 in helium flow at 150° C. for 90 minutes. Results are shown in FIG. 5. By performing thermal desorption spectroscopy during pretreatment, some removal of water was observed, which is consistent with the weight loss observed using thermogravimetric analysis. The first cycle of NO adsorption was then performed at 50° C., by treating 1 with a helium flow containing a fixed amount of NO and monitoring the amount of NO adsorbed by mass spectrometry on the effluent stream. The amount of NO adsorbed during this first cycle corresponded to 78 μmol / g or 38% of the possible number of sites. Temperature ramping up to 250° C. was subsequently performed on this sample, with the sample mai...
example 3
Vapor Phase Adsorption of Benzene and Cyclohexane
[0059] Since immobilized calixarenes on silica are known to form complexes with neutral molecules, we explored the vapor-phase adsorption of guest to 1. The adsorption of benzene and cyclohexane was performed from the vapor phase, by contacting 1 with benzene and cyclohexane gas and measuring the uptake from the consequent decrease in the pressure of the contacting gas phase. FIG. 6 shows the amount of benzene and cyclohexane adsorbed onto 1 at 70° C. as the driving force for adsorption, measured by the relative pressure (pressure divided by the vapor pressure Po at the adsorption temperature), is varied. These data show that cyclohexane adsorption uptakes are significantly smaller than those of benzene on 1. This may reflect in part the aromatic character of benzene molecules and their ability to interact specifically with the π-electrons in the aromatic environment provided by the immobilized calixarenes. The total amount adsorbed ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com