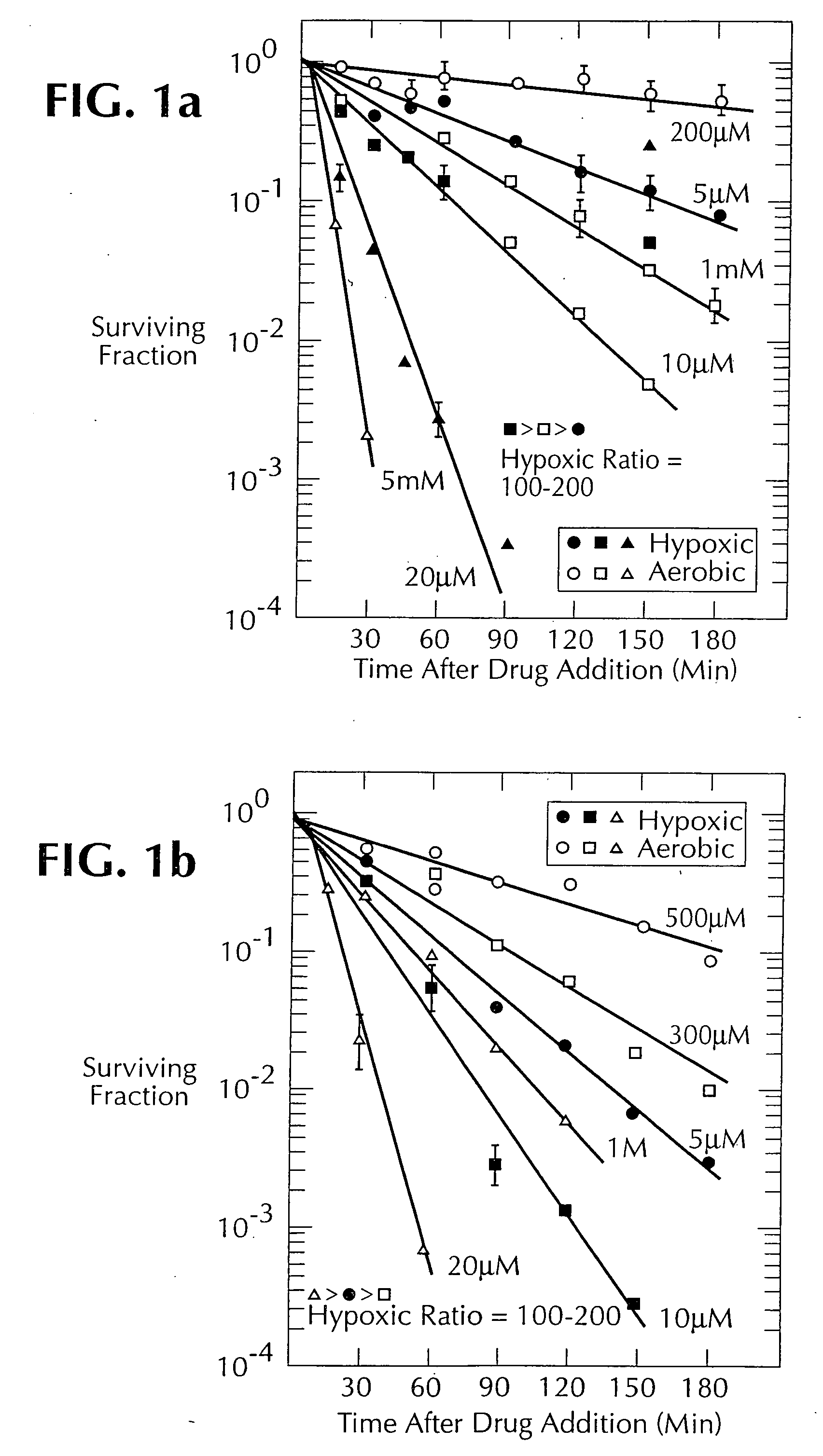
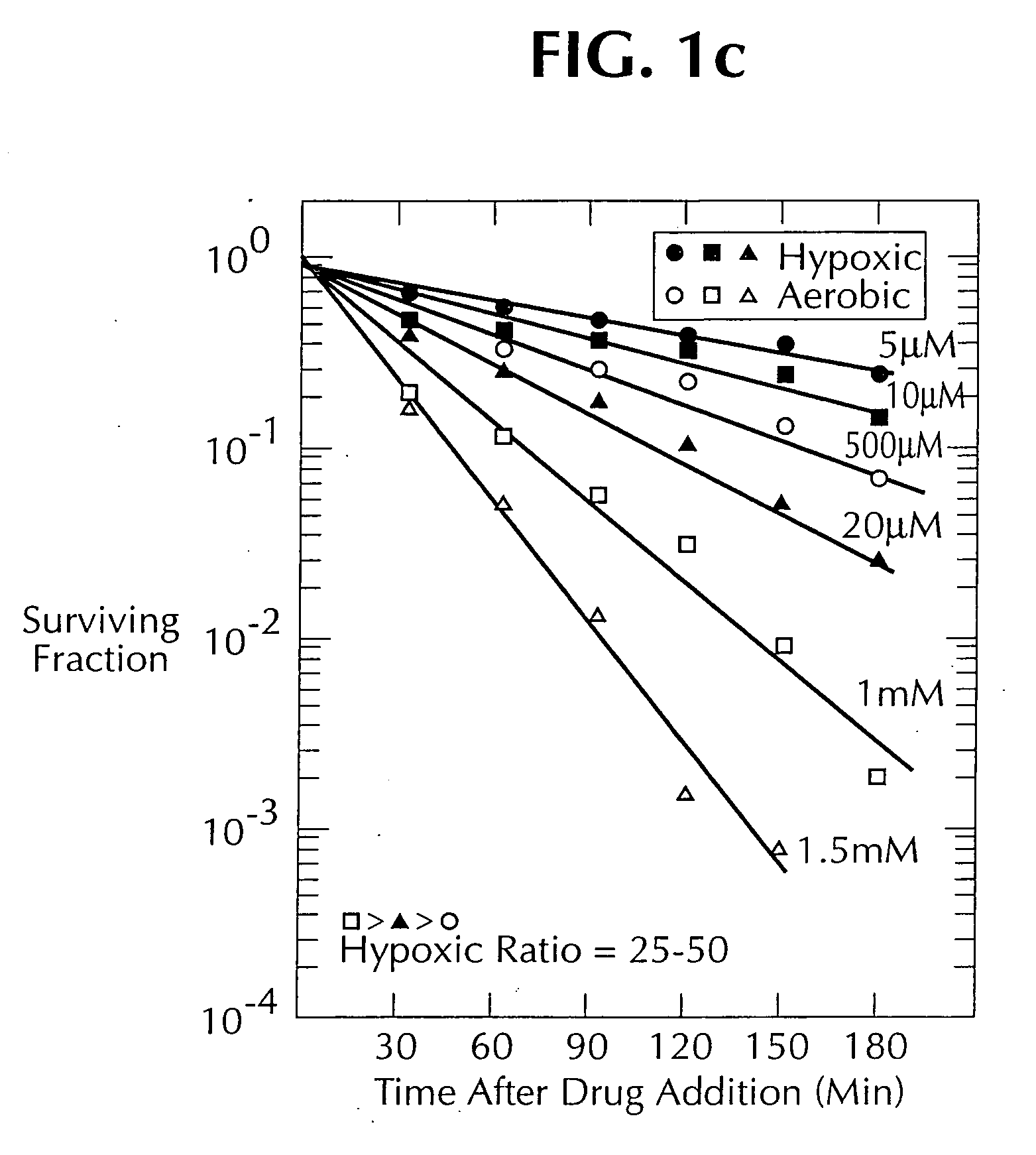
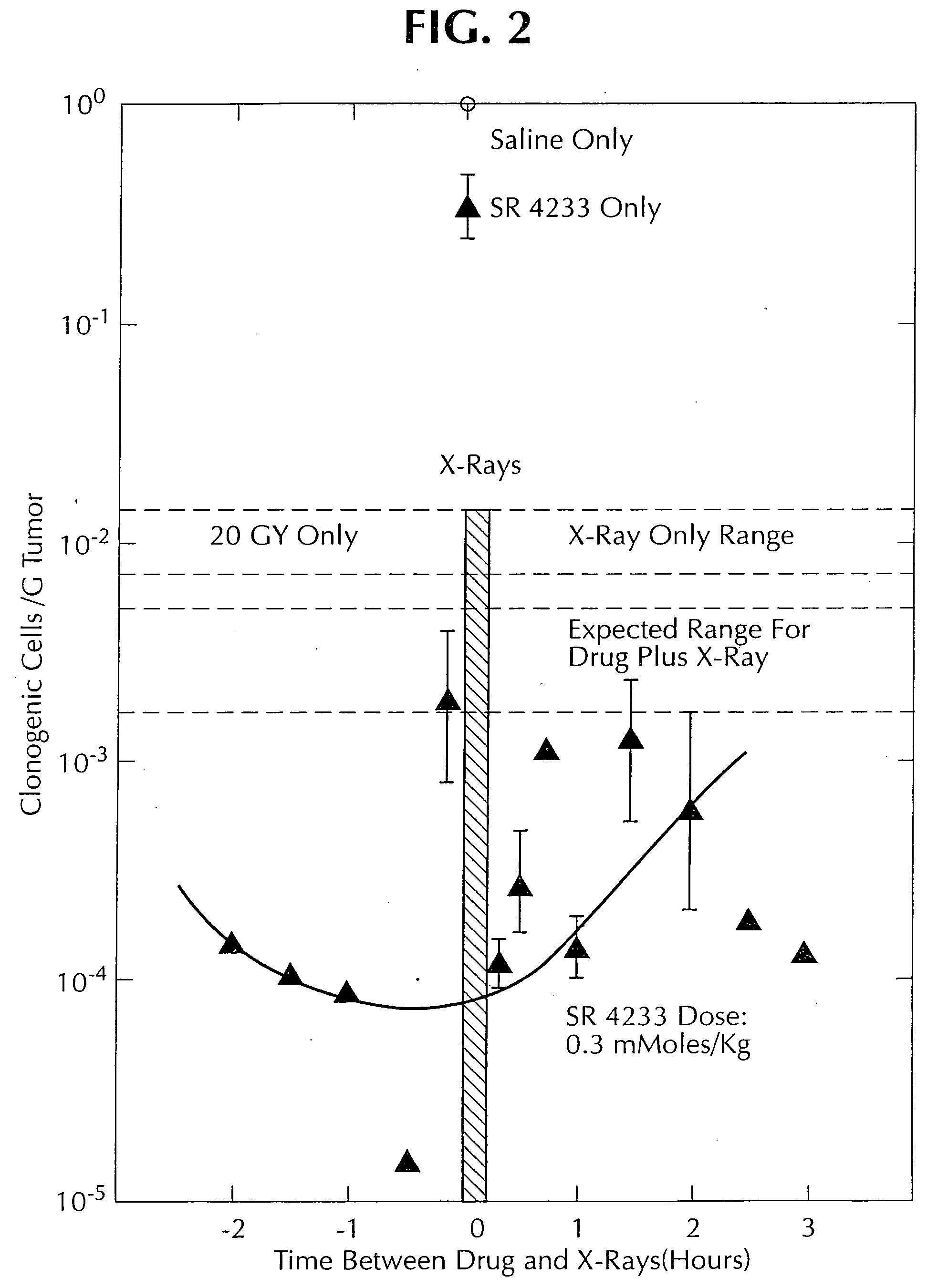
1, 2, 4-benzotriazine oxides as radiosensitizers and selective cytotoxic agents
a technology of benzotriazine oxide and radiosensitizer, which is applied in the direction of biocide, heterocyclic compound active ingredients, organic chemistry, etc., can solve the problems of only practical radiation treatment to destroy tumor cells, and difficult to achieve tumor cell selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
Preparation of 3-Hydroxy-1,2,4-Benzotriazine 1,4-Dioxide
[0217]
[0218] A stirred mixture of 1.50 g (9.25 mmole) of 3-amino-1,2,4-benzotriazine 1-oxide (1), 100.0 ml acetic acid, and 30.0 ml of 30% hydrogen peroxide was treated with 3.05 g (9.25 mmole) of Na2WO4 2H2O. The mixture was stirred in an oil bath at 60° C. for 4 days. The yellowish orange mixture was cooled to about 30° and filtered to remove a light yellow non-UV absorbing solid. The orange solution of hydrogen peroxide in acetic acid was evaporated to semi-dryness carefully with several additions of water and acetic acid to remove most of the peroxide. The concentrated solution was allowed to stand at room temperature to afford four crops of an orange solid, 0.87 g (42% yield of the sodium salt of 2). UVmax (20% CH3OH / H2O): 262.2 (ε 39,460); 477 (ε 7,030)., IR (neat): 3530μ, 3150μ, 2650μ, 2180μ and 1635μ. Anal. (calculated for the sodium salt): C7H4N3O3Na 1.25H2O, 223.64: C, 37.6; H, 2.93; N, 18.79. Found: C, 37.8; H, 2.75...
example 2
Preparation of 3-Amino-7-Trifluoromethyl-1,2,4-Benzotriazine 1-Oxide
[0219]
[0220] A mixture of 4-chloro-3-nitrobenzotrifluoride (Aldrich, 2.70 g, 12.9 mmole) and cyanamide dihydrochloride (2.75 g, 24 mmole) (previously prepared by treating an ether solution of cyanamide with HCl gas and collecting the precipitated solid) was heated at 140° C. for 1 h. The residue was treated with 2N NaOH (45 ml), heated for a further 5 min, and then allowed to cool. The precipitate was collected, washed with H2O, dried, and triturated with acetone-toluene to yield 1.32 g (45%) of 3 as a light yellow solid M.P. 301-302°, TLC: Rf 0.60 (9:1 methylene chloride:methanol on silica gel plates). Mass. Spec.: M+=230 (q=100).
example 3
Preparation of 3-Amino-7-Decyl-1,2,4-Benzotriazine 1-Oxide
[0221]
[0222] Preparation of 4-(1-decyl)-2-nitroaniline: Acetic anhydride (400 ml) was added over a 30-minute period to a stirred solution of 4-decylaniline (Aldrich, 80 g, 0.34 mole) in hexanes (2.41). After stirring for 1 h, the mixture was cooled and treated over 30 min. at 5-10° C. with 70% nitric acid (34 ml). Stirring was continued at 5-10° C. for 1 h and at 25° C. for 16 h. The mixture was diluted with H2O (11), stirred for 5 h, poured into an open dish and allowed to stand for 16 h. After further dilution with H2O (1.51), the solid was collected and recrystallized from an 85% ethanol solution (in water) to give 92 g (84%) of the intermediate as an orange solid, m.p. 64° C.
[0223] A solution (100 ml) of 85% KOH (19 g, 0.288 mole) in H2O was combined with a suspension of 4-(1-decyl)-2-nitroaniline (89 g, 0.28 mole), prepared above, in methanol (900 ml). The mixture was stirred for 6 h, neutralized to pH 7-8 with concent...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| Mass. Spec | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com