Systems and methods for treating fractured or diseased bone using expandable bodies
a technology of expandable bodies and fractured or diseased bone, which is applied in the field of bone conditions treatment, can solve the problems of chronic complications, less interior support for more prone to compression fracture or collapse of the surrounding cortical bone,
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
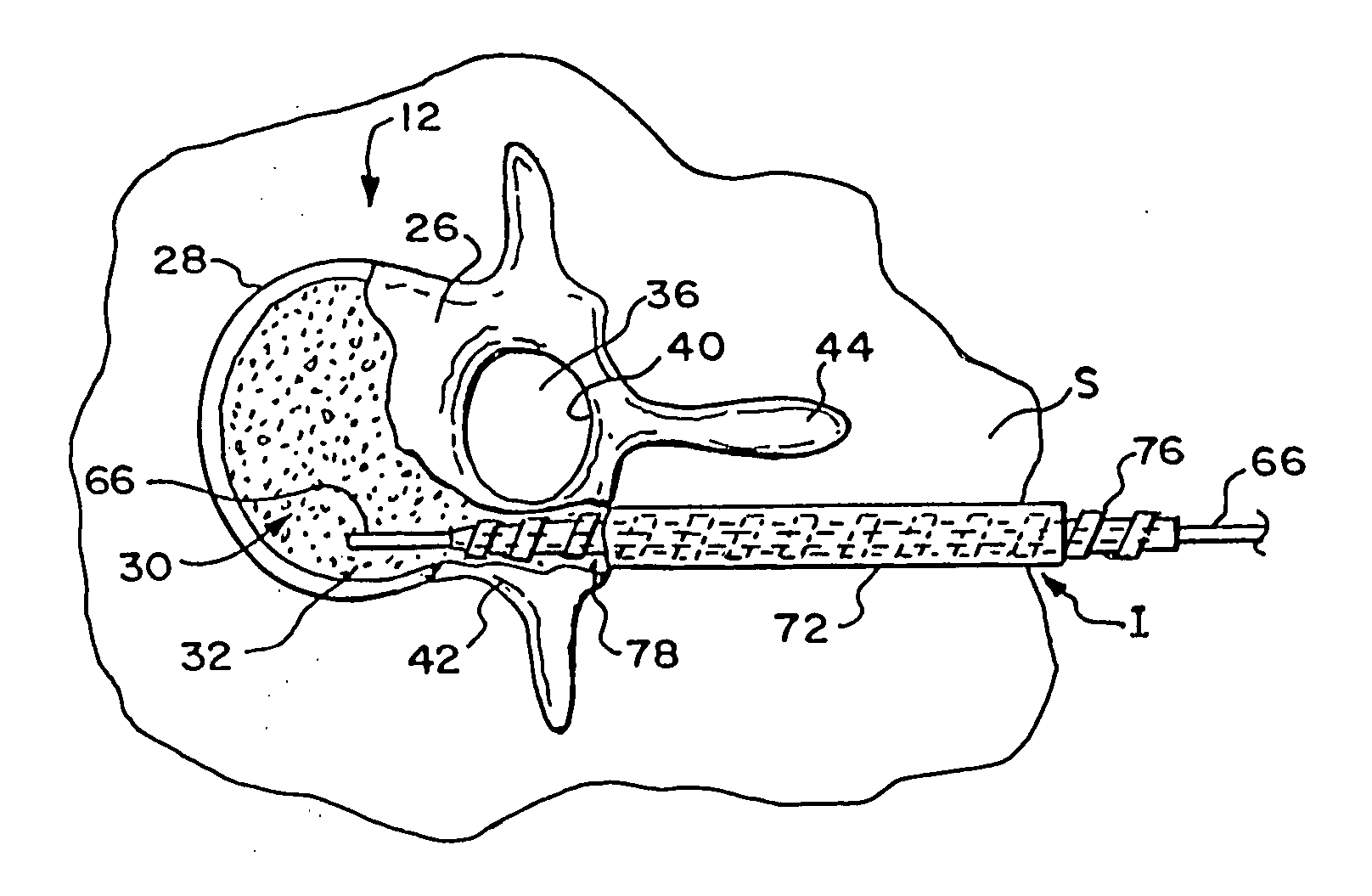
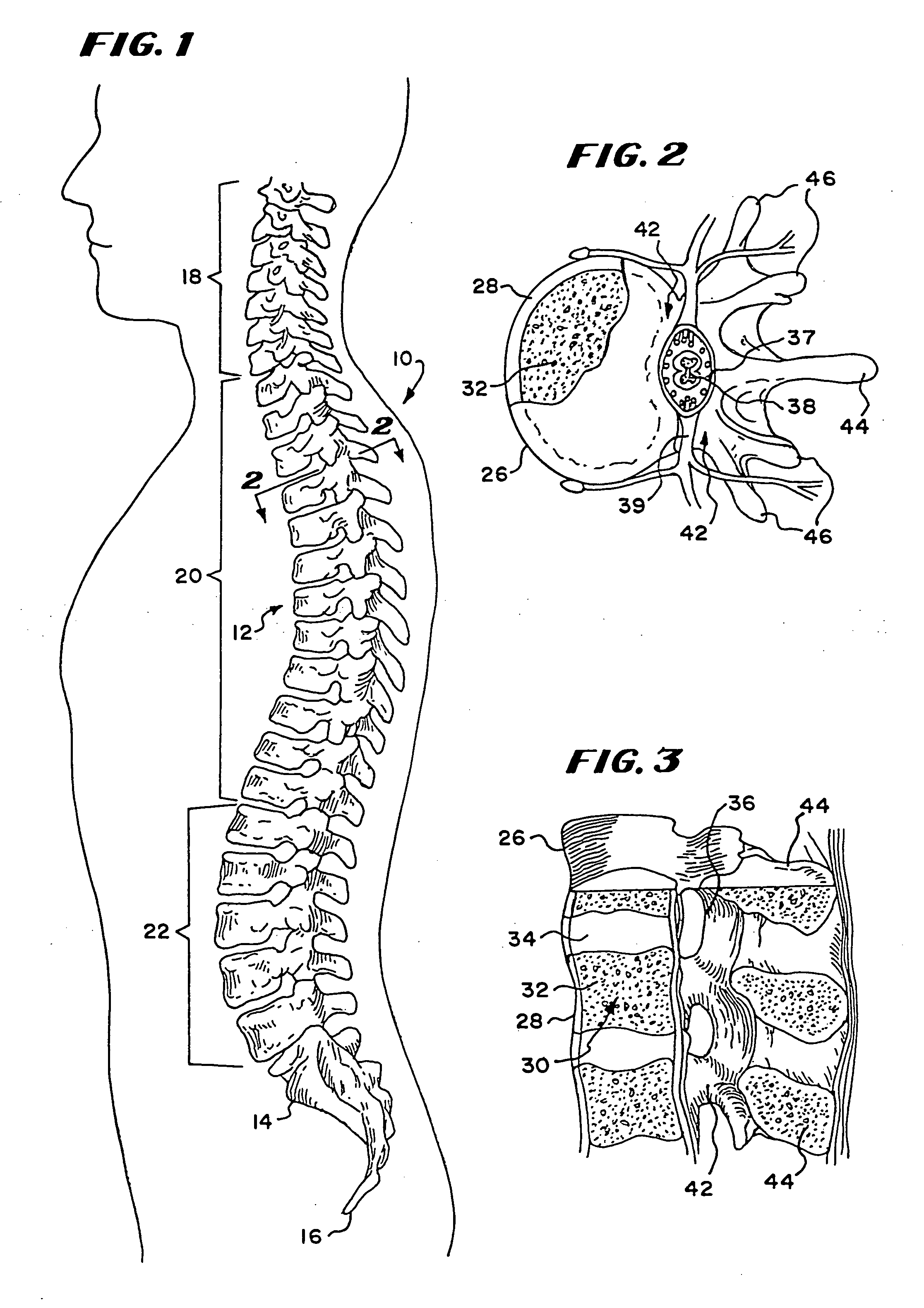
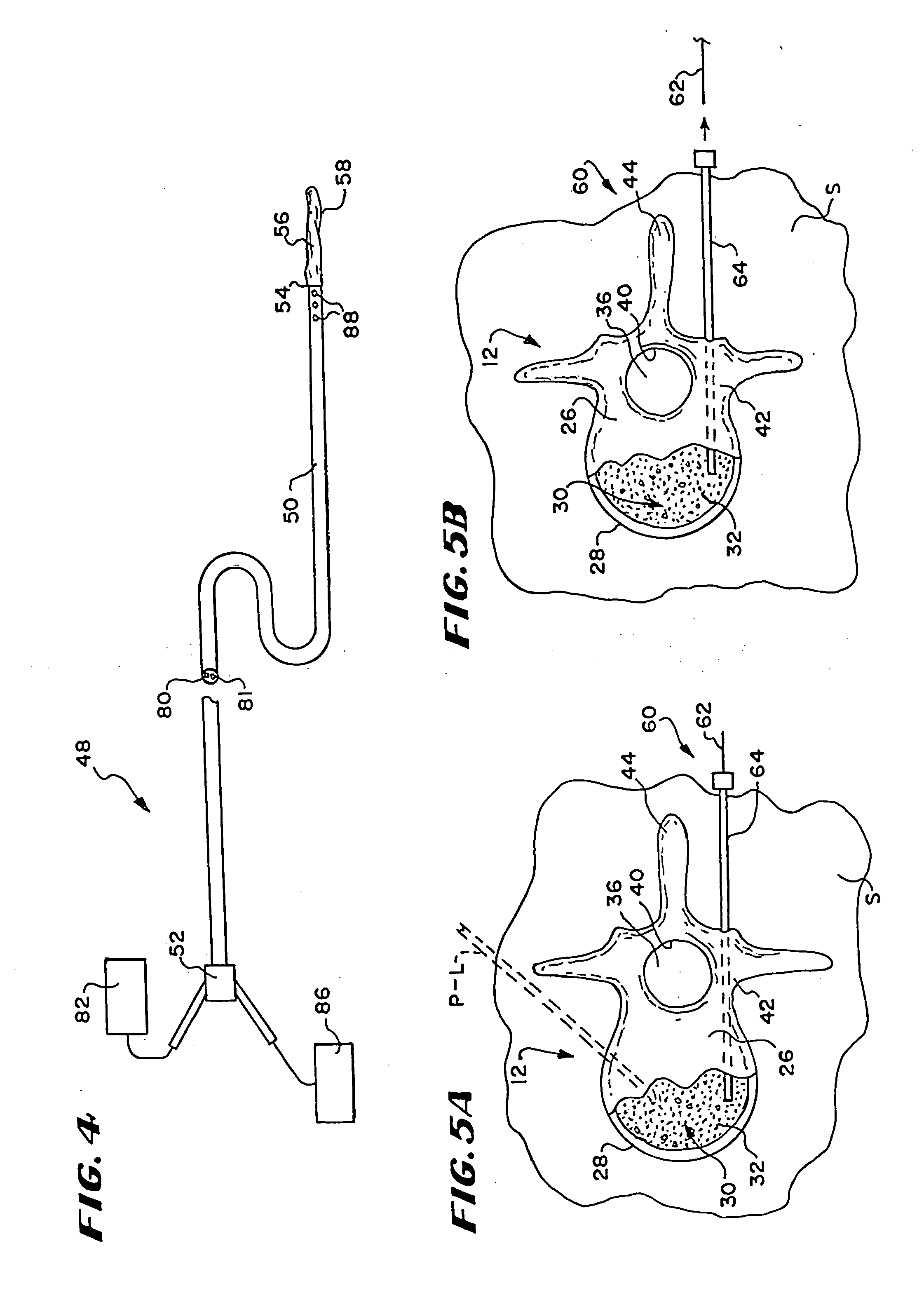
[0074] This Specification describes new systems and methods to treat bones using expandable bodies. The use of expandable bodies to treat bones is disclosed in U.S. Pat. Nos. 4,969,888 and 5,108,404, which are incorporated herein by reference. Improvements in this regard are disclosed in U.S. patent application Ser. No. 08 / 188,224, filed Jan. 26, 1994; U.S. patent application Ser. No. 08 / 485,394, filed Jun. 7, 1995; and U.S. patent application Ser. No. 08 / 659,678, filed Jun. 5, 1996, which are each incorporated herein by reference.
[0075] The new systems and methods will be first described with regard to the treatment of vertebra. It should be appreciated, however, the systems and methods so described are not limited in their application to vertebrae. As will be described in greater detail later, the systems and methods are applicable to the treatment of diverse bone types.
[0076] I. Treatment of Vertebral Bodies
[0077] As FIG. 1 shows, the spinal column 10 comprises a number of uni...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| pressures | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com