Treatment of neurological conditions using complement c5a receptor modulators
A technology of receptor inhibitors and diseases, applied in the direction of nervous system diseases, cardiovascular system diseases, anti-inflammatory agents, etc., can solve problems such as no evidence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0181] Example 1 A pilot study of the role of PMX53
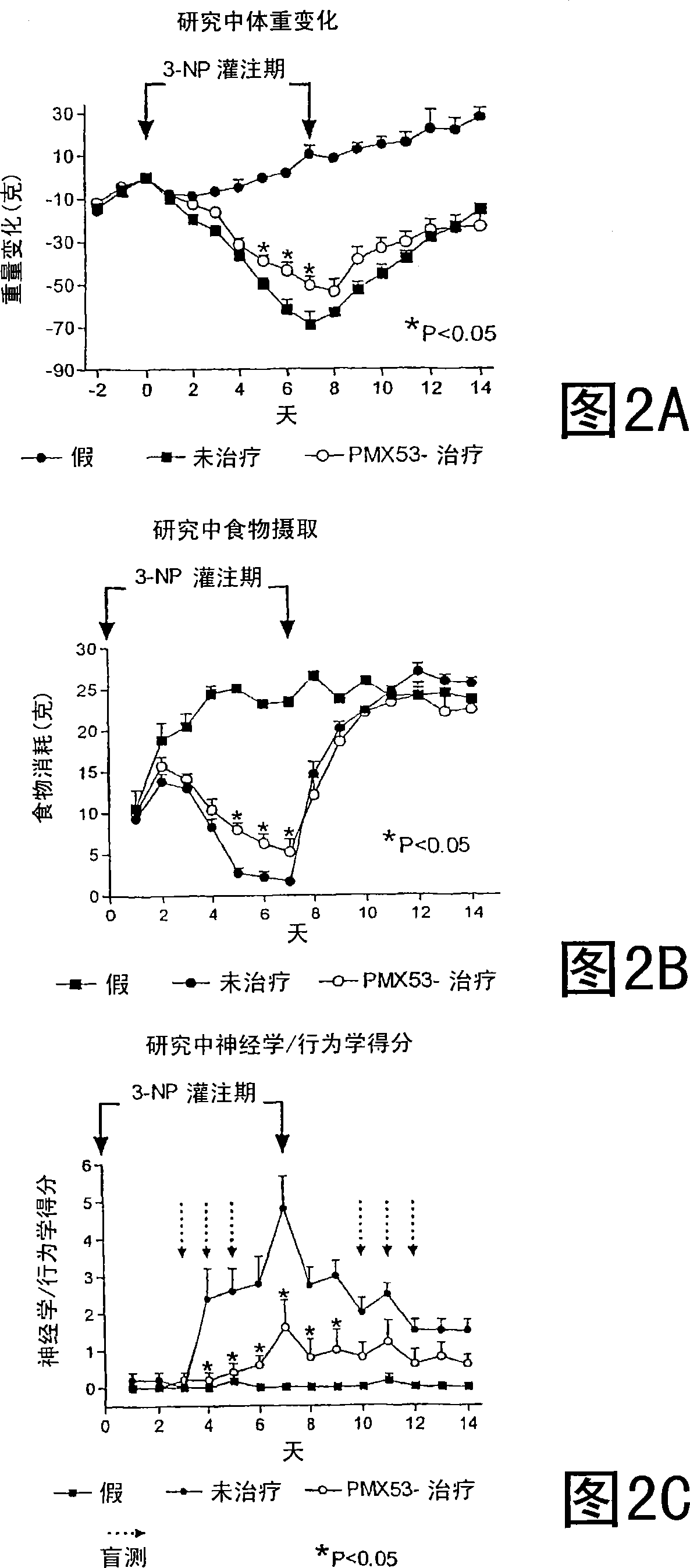
[0182] Preliminary experiments have shown that a dose of 42 mg / kg / day administered for 7 days reproducibly induces this model. To test the effect of PMX53 in this model system, a pilot study was performed. In this study, rats treated with PMX53 were compared to sham-operated and untreated controls. A total of 12 rats were used as follows:
[0183] fake operation 2
[0184] Untreated 5
[0185] PMX53 5
[0186] Daily administration of PMX53 with drinking water at a dose of 2 mg / kg was started two days before 3-NP administration. These PMX53-treated animals were given a dose of 1 mg / kg s.c. on days 0, 3, 6 and 8, and since they were not eating, it was considered possible that they were not drinking. In subsequent experiments, animals were given daily doses by gavage, starting on day 2, to avoid this potential influencing factor. In this initial experiment, the pump was removed after 7 days and the skin was sutured ...
Embodiment 2
[0190] Example 2 Use other substances to compare
[0191]The effect of PMX53 was compared with another compound of formula I, PMX205 (hydrocinnamic acid-[OpdChaWR](HC-[OPdChaWR]) and the known anti-inflammatory agents ibuprofen and infliximab. The groups of rats and the number of each group were as follows :
[0192] fake operation 4
[0193] Untreated 6
[0194] PMX53 4
[0195] PMX205 4
[0196] ibuprofen 5
[0197] Infliximab 4
[0198] PMX53 (10 mg / kg / day) and PMX205 (10 mg / kg / day) were administered daily by gavage and ibuprofen (30 mg / kg / day) was administered in drinking water, starting two days before 3-NP administration. Infliximab was administered intravenously as a single dose of 5 mg / kg on day 0.
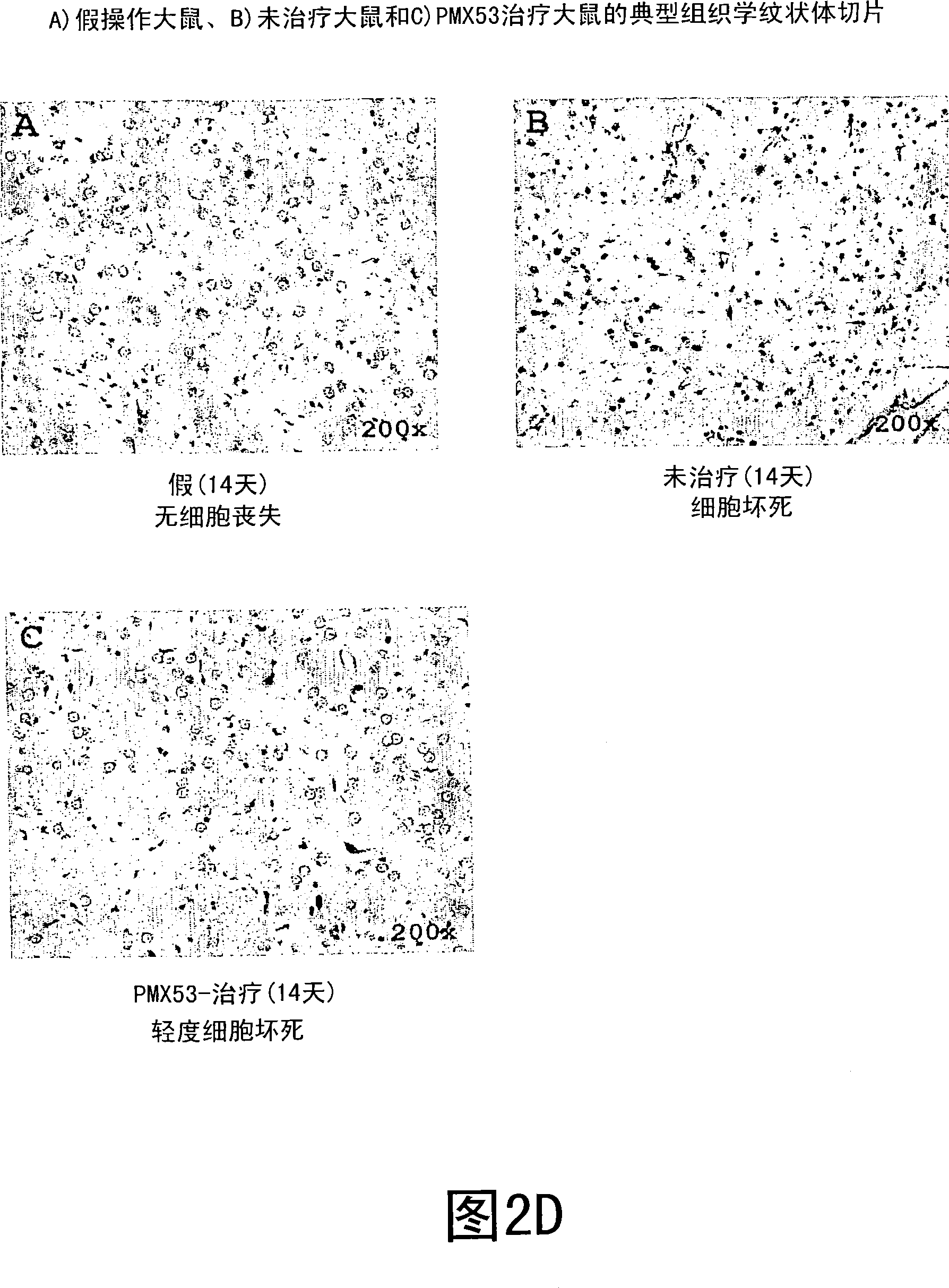
[0199] The results are shown in Figures 3A-3D. Figures 2A and 2B show that the extent of body weight gain and food consumption after 7 days is similar to that observed in Example 1. For neurologic / behavioral scores, both PMX53 and PMX205 had significant protec...
Embodiment 3
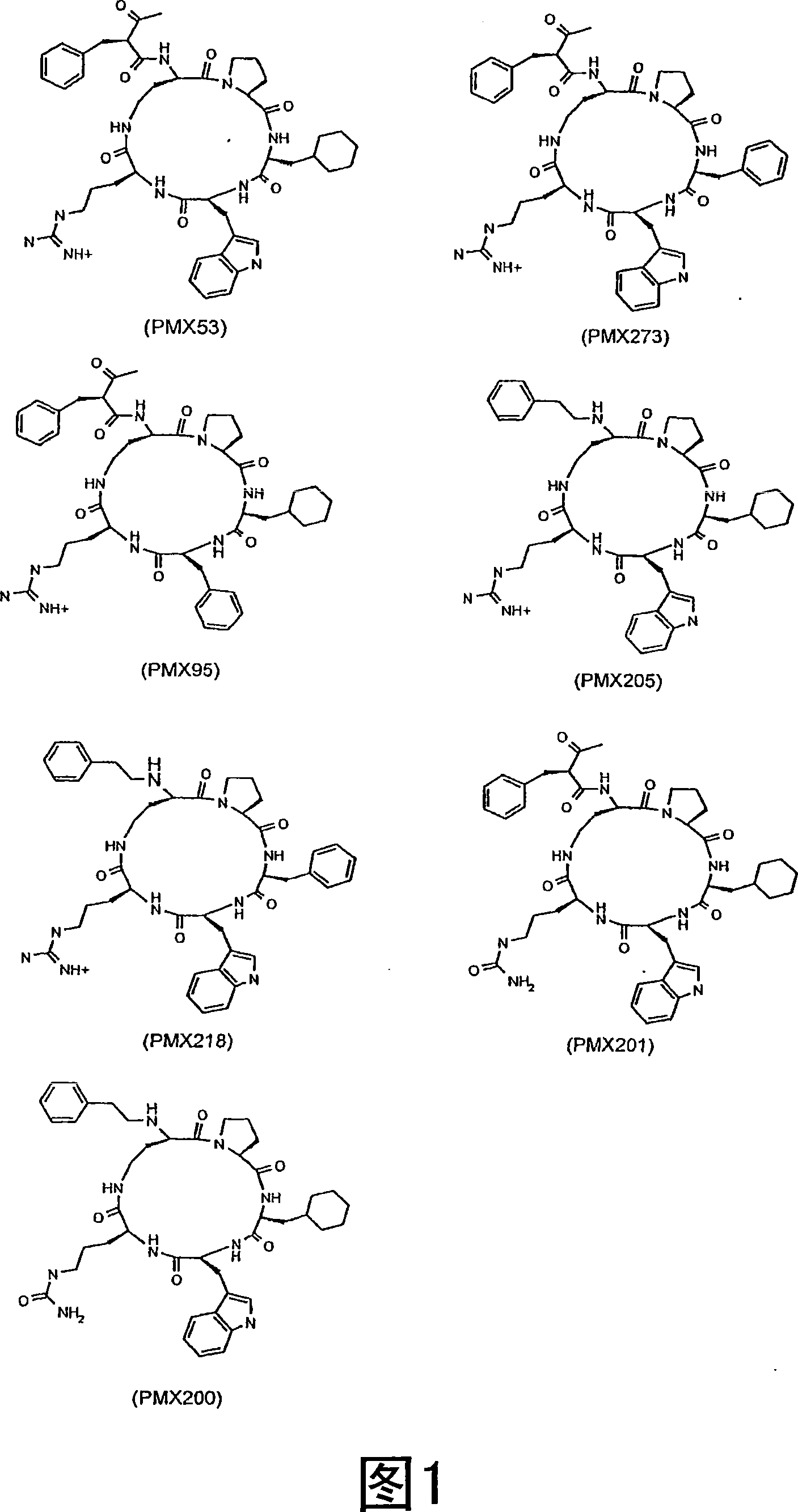
[0209] Example 3 Effects of PMX53 analogues
[0210] The following compounds of formula I were tested in the same manner as described in Example 2:
[0211] PMX205: HC-[OPdChaWR]
[0212] PMX273: AcF-[OPdPheWR]
[0213] PMX201: AcF-[OPdChaW citrulline]
[0214] PMX218: HC-[OPdPheWR]
[0215] Starting on day 2, all drugs were administered by gavage at a dose of 10 mg / kg / day. If this dose is found to be effective, the 3 mg / kg / day and 1 mg / kg / day or less doses are retested in order to determine the dose-response relationship. The dose-response relationship of PMX53 was also determined.
[0216] The effects of these substances were also compared with those of infliximab (infliximab (in a single dose of 5 mg / kg iv on day 0) and ibuprofen (30 mg / kg) given through drinking water.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com