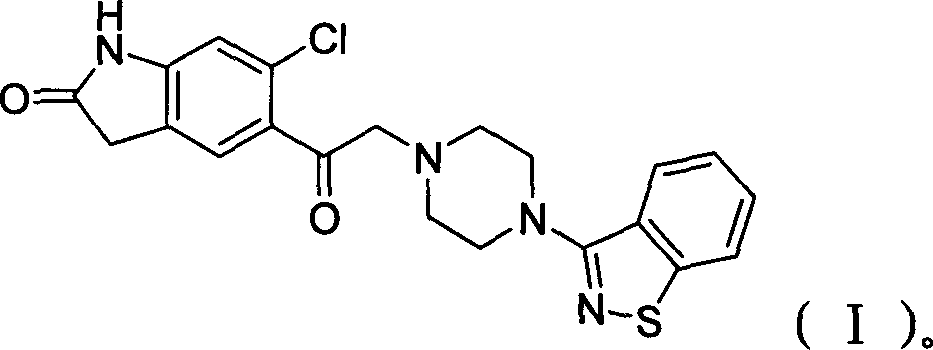
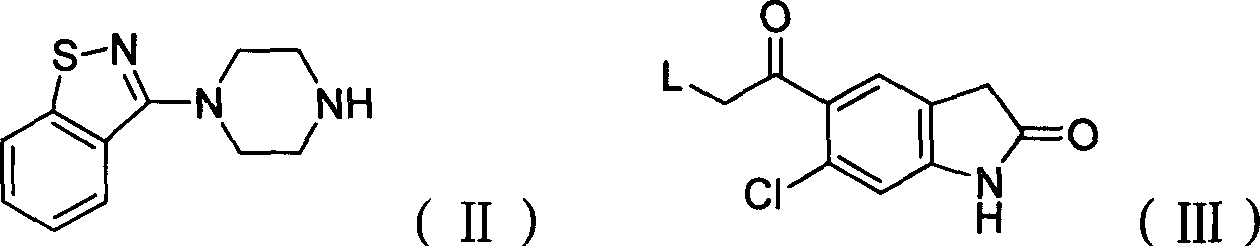Preparation method of ziprasidone
A technology of ziprasidone and benzisothiazole, which is applied in the field of preparation of ziprasidone, can solve the problems of long reaction time, low preparation yield, complicated operation, etc., and achieve short reaction time, simple operation and high yield high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Embodiment 1 (comparative example):
[0031] 0.73g (3.20mmol) 5-(2-chloroethyl) 6-chloro-indolinone, 0.70g (3.2mmol) N-(1,2-benzisothiazol-3-yl) piperazine, 0.68g (.40mmol) sodium carbonate, 2mg sodium iodide, 30ml methyl isobutyl ketone, join in the 125ml round bottom flask that nitrogen inlet and condenser are equipped with, react reflux 40hr, cool, filter, distill. The distillate was purified by column chromatography, the by-product was eluted with 11 ethyl acetate, the product was eluted with 1.51 ethyl acetate containing 4% methanol, the washed product was distilled, the distillate was added to dichloromethane, and then added Diethyl ether was saturated with hydrochloric acid gas, resulting in precipitation, filtered, washed with ether, filtered, and the filter cake was washed with acetone, filtered to obtain ziprasidone hydrochloride with a yield of 20%.
Embodiment 2
[0032] Example 2: 5-(2-(4-(1,2-benzisothiazol-3-yl)-piperazine)acetyl)-6-chloro-1,3-dihydro-2H-indole- Preparation of 2-keto:
[0033] Add 7.9 g (31 mmol) of N-(1,2-benzisothiazol-3-yl) piperazine hydrochloride into a 250 ml three-necked flask, add 40 ml of DMF (dimethylformamide), and stir. Add 4.88g (20mmol) of 5-(2-chloroacetyl) 6-chloro-indolinone, 6g of potassium iodide, and 10g of potassium carbonate, and start to heat up to 70°C. After 4 hours of heat preservation, distill under reduced pressure, add 40ml of water, and add 6N hydrochloric acid, adjust the pH to 3-4, filter, wash the filter cake with water until neutral, and dry at 55°C to obtain 7.21g of product, yield: 82.5%. 5-(2-(4-(1,2-Benzisothiazol-3-yl)-piperazine)acetyl)-6-chloro-1,3-dihydro-2H-indol-2-one Physical parameters:
[0034] MS(%) M +1 426+1
[0035] NMR (d, CDCl 3 ): 3.2-3.6(m, 8H), 7.4-7.7(m, 2H), 7.8(d, 1H), 7.87(d, 1H), 2.7(s, 2H), 6.9(s, 1H), 10.8( s,1H), 3.8(s,2H), 7.7(s,1H)
Embodiment 3
[0036] Example 3: 5-(2-(4-(1,2-benzisothiazol-3-yl)-piperazine)acetyl)-6-chloro-1,3-dihydro-2H-indole- Preparation of 2-keto:
[0037] Add 6.8 g (31 mmol) of N-(1,2-benzisothiazol-3-yl) piperazine into a 250 ml three-neck flask, add 30 ml of DMF (dimethylformamide), and stir. Add 4.88g (20mmol) of 5-(2-bromoacetyl)6-chloro-indolinone, 7g of sodium iodide, and 11g of sodium bicarbonate, start to heat up to 50°C, keep warm for 12 hours, distill under reduced pressure, and add 40ml Add water, add 6N hydrochloric acid, adjust the pH to 3-4, filter, wash the filter cake with water until neutral, and dry at 55°C to obtain 6.02g of product, yield: 68.9%. Example 4: 5-(2-(4-(1,2-benzisothiazol-3-yl)-piperazine)acetyl)-6-chloro-1,3-dihydro-2H-indole- Preparation of 2-keto:
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com