Process for preparing atazanavir bisulfate and novel forms
A technology of bisulfate and atazanavir, applied in the field of preparing HIV protease inhibitor atazanavir bisulfate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
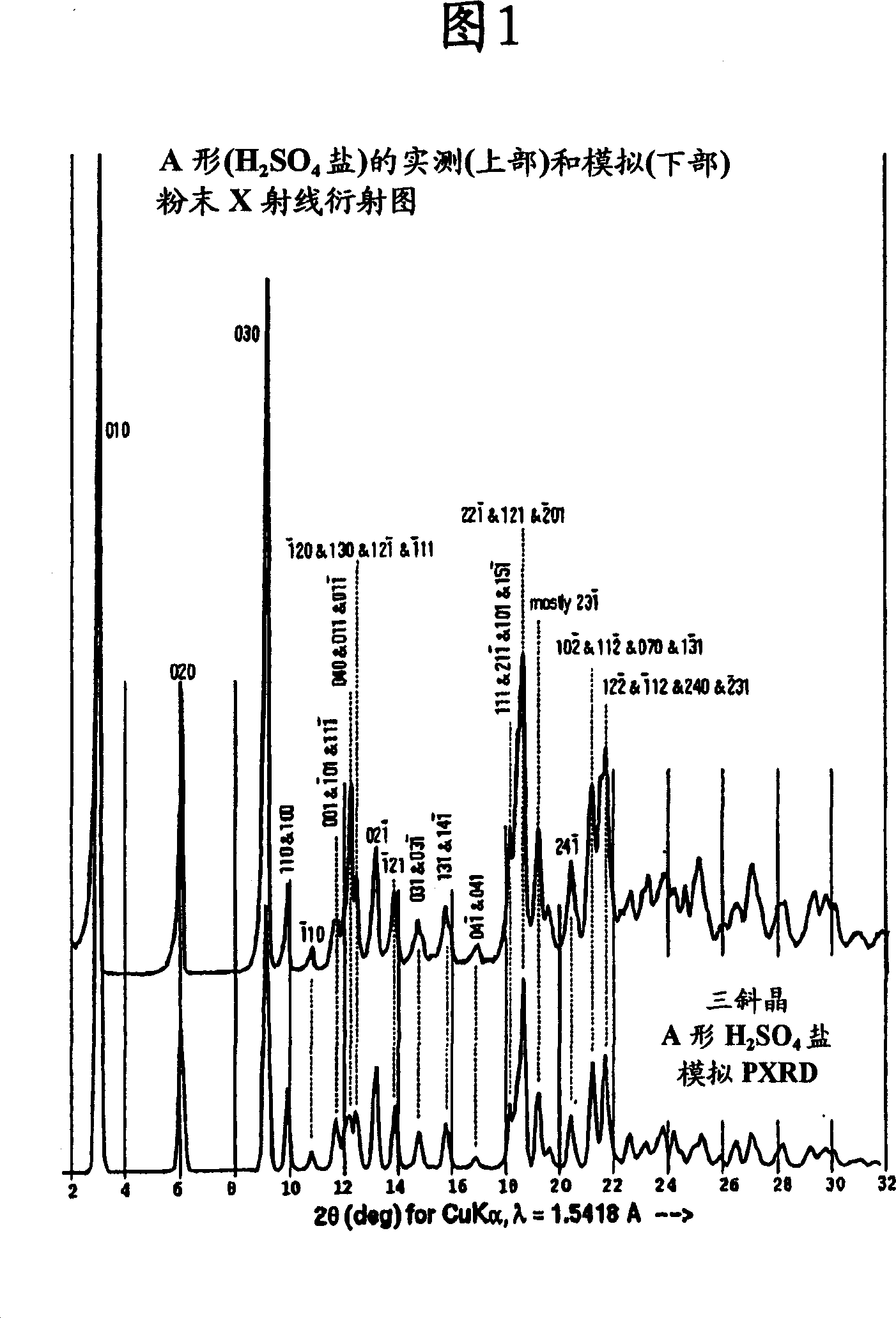
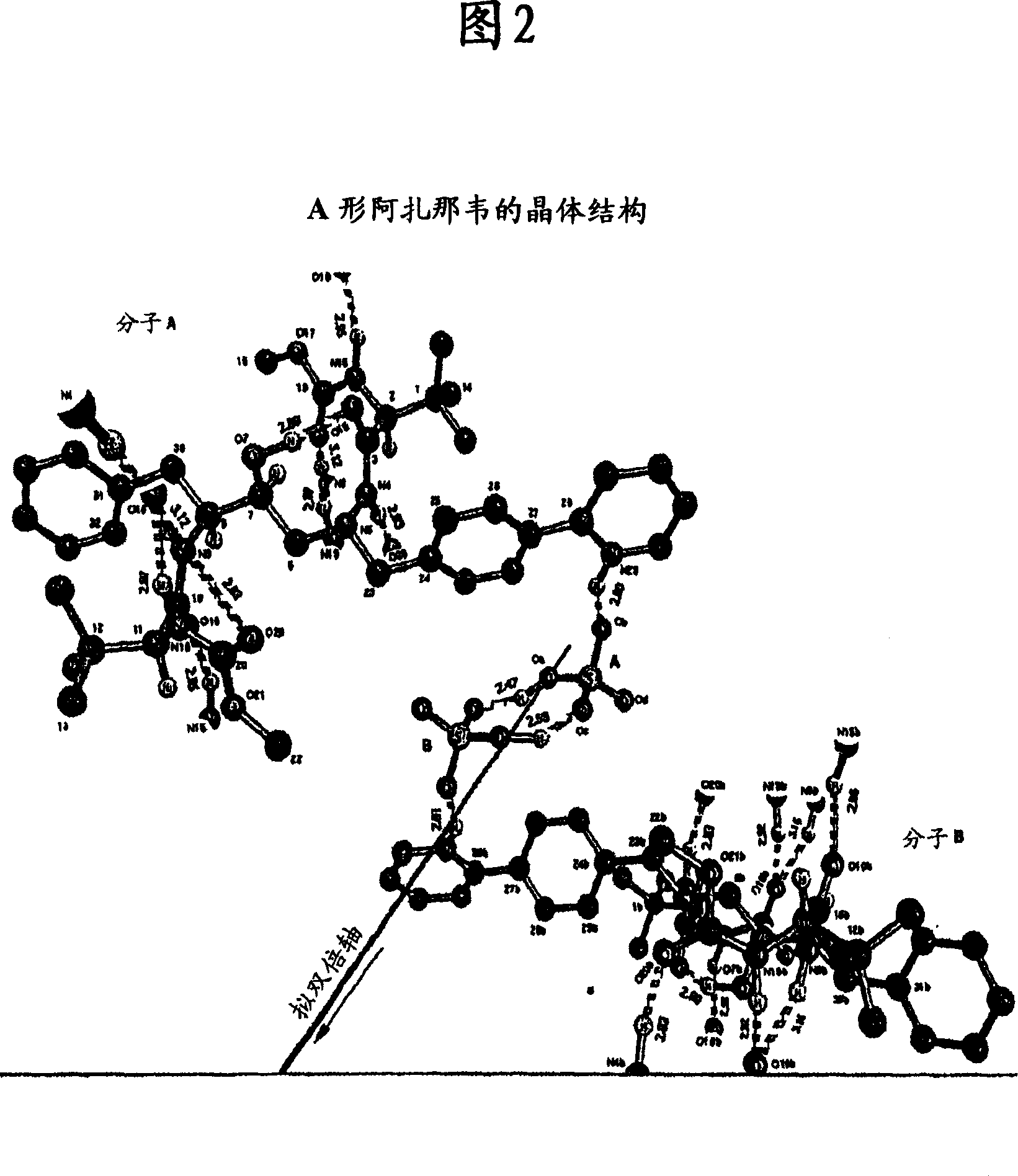
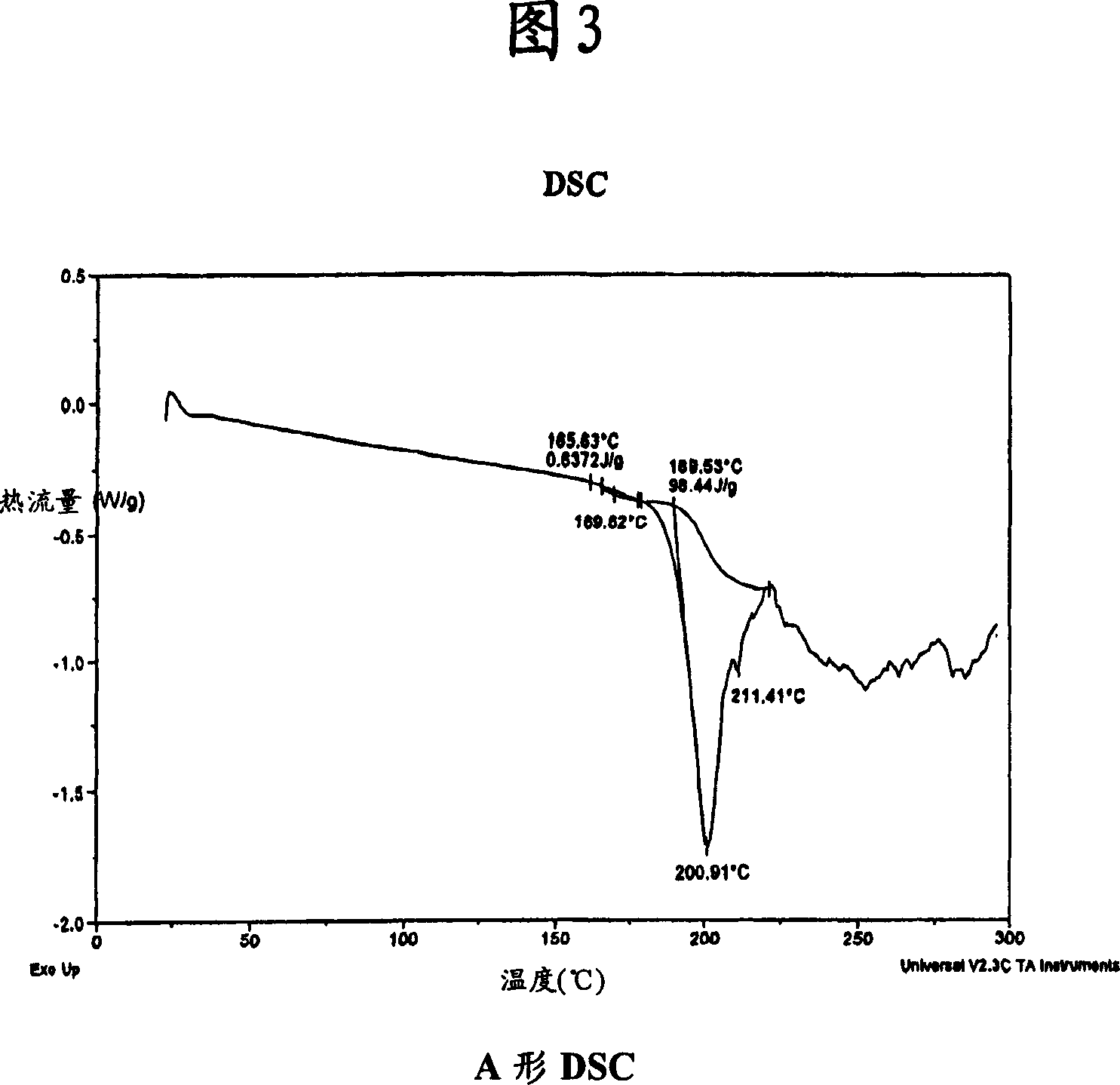
[0177] 1-[4-(pyridin-2-yl)phenyl]-5(S)-2,5-bis{[N-(methoxycarbonyl)-L-tert-leucyl]amino}-4-( S)-Hydroxy-6-phenyl-2-azahexane, bisulfate (Form A) (Atazanavir bisulfate-Form A)
[0178] a.
[0179]
[0180] (1-[4-(pyridin-2-yl)phenyl]-5(S)-2,5-bis[tert-butoxycarbonyl)amino]-4(S)-hydroxy-6-phenyl-2 -Azahexane.3HCl (triamine.3HCl salt))
[0181] The protected triamine 1-[4-(pyridin-2-yl)phenyl]-5(S)-2,5-bis[tert-butoxycarbonyl)amino]-4(S)-hydroxyl-6 -Phenyl-2-azahexane
[0182]
[0183] (100g, 0.178mol) and CH 2 Cl 2 (500 mL; 5 mL / g protected triamine feed) (its preparation is described in Z. Xu et al., Process Research and Development for an Efficient Synthesis of the HIV Protease Inhibitor BMS-232, 632, Organic Process Research and Development, 6, 323-328 (2002)) into a 1000 mL 3-necked round bottom flask equipped with a mechanical stirrer, nitrogen inlet, and temperature sensor, and the resulting slurry was stirred while maintaining the temperature at about 5 to abo...
Embodiment 2
[0233] Atazanavir bisulfate-C pattern substance
[0234] Method A:
[0235] Atazanavir bisulfate Form A crystals (prepared as described in Example 1) (25.33 g) were suspended in 200 mL of water, and the mixture was mechanically stirred to give a viscous gel, which was dried.
[0236] The dry mixture was triturated with a spatula to obtain the Pattern C material. The powder X-ray diffraction pattern of the C-mode substance is shown in FIG. 6 .
[0237] Method B:
[0238] Wet granulate the atazanavir bisulfate Form A crystals with sufficient water (approximately 40% w / w) in a suitable mixer-granulator. Dry the wet mass in an oven. The product is sieved to size using a suitable sieve. The X-ray diffraction pattern of the obtained product was consistent with that of the C-mode material shown in FIG. 6 .
[0239] The differential scanning calorimetry thermogram characteristic of the C mode is shown in Fig. 7, and generally there is an endotherm in the range of about 76.7 to a...
Embodiment 3
[0242] Atazanavir Bisulfate-Form E3 (Triethanol Solvate)
[0243] In a 100mL 3-necked round-bottomed flask equipped with a mechanical stirrer, a temperature sensor and a pressure-balanced addition funnel, atazanavir free base (prepared according to Part C in Example 1) (3.0g, 4.26mmol) was dried at 200 Slurry was made in proof ethanol (20.25 mL, 6.75 mL / g free base).
[0244] Concentrated H 2 SO 4(0.25 mL, 0.46 g, 4.69 mmol, 1.1 eq.) was added to the atazanavir free base slurry maintained at 20-25 °C. The resulting solution (KF 0.2 to 1.0% water) was polish filtered (Whatman #1 paper), the filter was rinsed with 2.25 mL of absolute ethanol, and the rinse was added to the filtered solution. The solution was heated to 37°C, seeded with 10 mg of amorphous atazanavir bisulfate derived from Form E3 crystals (by exposing Form E3 crystals to ambient temperature), and the mixture was stirred for 15 minutes. Heptane (380 mL, 8.25 mL / g free base) was added over 1 hour. The resultin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com