Degradable implated medicine release-controlling carrier with micropores and cavities and its preparing process
A drug controlled release, multi-chamber technology, applied in the field of medicine and chemical industry, can solve the problems of patient pain, inability to realize the release cycle, reduce the drug release time of the drug burst effect, and improve the initial drug release stagnation and mid-term burst release. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
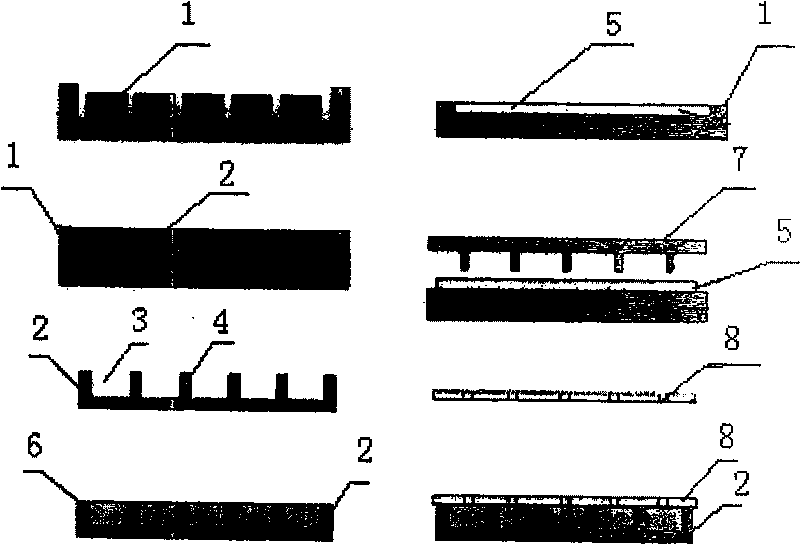
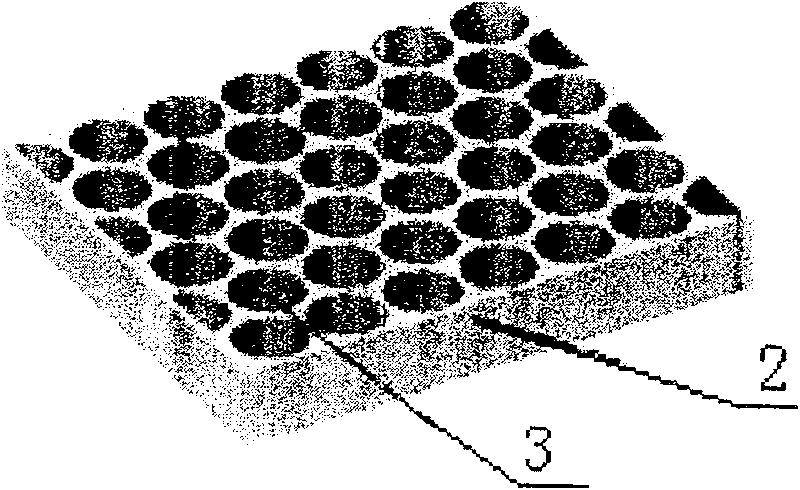
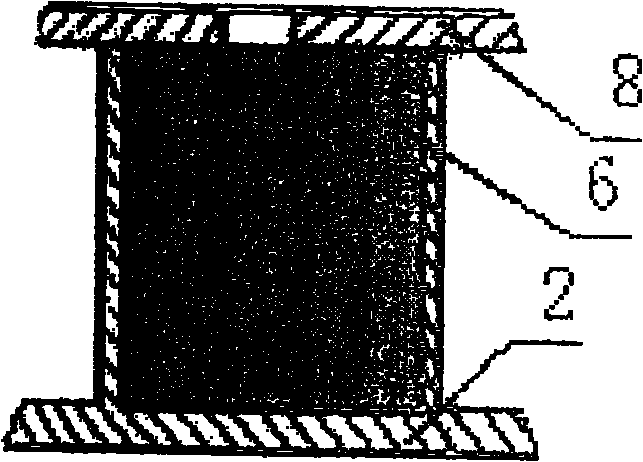
[0015] Example 1, see figure 1 , first use deep ultraviolet lithography and electroplating process UV-LIGA to process polydimethylsiloxane (PDMS) mold 1 for preparing carrier on silicon wafer; then use evaporation solvent method to prepare polylactic acid-polyhydroxy Acetic acid copolymer, polylactic acid or polyanhydride drug-controlled release carrier 2 and encapsulation film 5, the drug-loaded cavity 3 of the drug-controlled release carrier 2 has a structural size of 500-2000 μm and a depth of 50-2000 μm, two drug-loaded cavities The distance between 3, that is, the wall thickness 4 is 10-30 μm figure 2 , the thickness of the packaging film 5 is 50-200 μm; for the preparation of the carrier structure, injection molding or molding method, especially the molding method, can also be used to prepare the corresponding mold, which can be mass-produced quickly and with high efficiency; the drug 6 Put it in the drug-loading cavity 3 of the drug-controlled release carrier 2 and cl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| depth | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com