Naphthylene derivatives as cytochrome P450 inhibitors
An alkyl and alkenyl technology, applied in the field of novel heteroaryl-naphthyl-alkylamines and salts thereof, can solve the problems of lack of specificity, insufficient consideration of activity/toxicity ratio, limitations and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
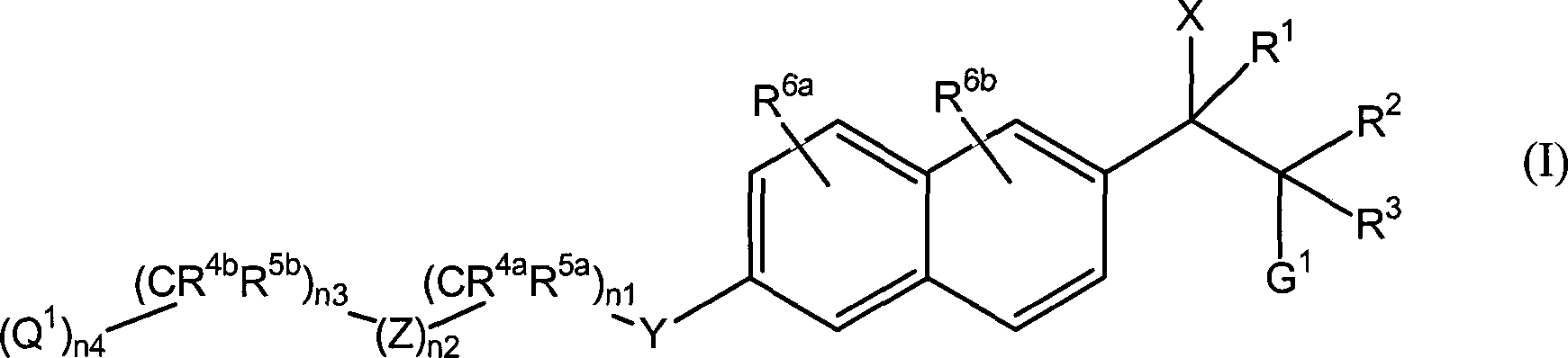
[0302] In a typical preparation, a compound of formula II is reacted with CDI or CDT in a suitable solvent. Suitable solvents for the above methods include, but are not limited to, ethers such as tetrahydrofuran (THF), glyme, etc.; dimethylformamide (DMF); dimethylsulfoxide (DMSO); acetonitrile; Such as dichloromethane (CH 2 Cl 2 ) or chloroform (CHCl 3 ). Mixtures of these solvents are used if desired. The preferred solvent depends on the substrate used and is chosen according to the nature of the substrate. The above process is carried out at a temperature of about -78°C to about 100°C. Preferably, the reaction is carried out at 22°C to about 80°C. The above-described methods of producing compounds of the invention are preferably carried out at about atmospheric pressure, although higher or lower pressures can be used if desired. Essentially, it is preferred to use equimolar amounts of the reactants, although higher or lower amounts can be used if desired.
[0303] C...
Embodiment 3-1a
[0596] Embodiment 3-1a (wherein X 1 = imidazol-1-yl, R 2 =CH 3 , R 3 = H, G 1 =N(CH 3 ) 2 , n 2 =0,n 3 = 1, R 4b and R 5b =CH 3 , n 4 = 1, and Q 1 =CO 2 CH 3 Compound of Formula I): The title compound was prepared according to General Synthetic Method D as described above, wherein in the compound of Formula II, R 2 =CH 3 , R 3 = H, G 1 =N(CH 3 ) 2 , n 2 =0,n 3 = 1, R 4b and R 5b =CH 3 , n 4 = 1, and Q 1 =CO 2 CH 3 . 1 H NMR (CDCl 3 , 200MHz) δ0.79(d, 3H, J=6.6Hz), 1.35(s, 6H), 2.27(s, 6H), 3.46-3.55(m, 1H), 3.70(s, 3H), 4.07(s , 2H), 5.05(d, 1H, J=10.6Hz), 7.00(s, 2H), 7.11-7.14(m, 1H), 7.17(d, 1H, J=5.2Hz), 7.26-7.30(m, 1H), 7.65 (d, 2H, J = 11.6 Hz), 7.72 (d, 2H, J = 8.8 Hz); MS (ES) 410.0 (M+1).
Embodiment 3-1b
[0597] Embodiment 3-1b (wherein X 1 = imidazol-1-yl, R 2 =CH 3 , R 3 = H, G 1 =N(CH 3 ) 2 , n 2 =0,n 3 = 1, R 4b and R 5b =CH 3 , n 4 = 1, and Q 1 =CO 2 CH 3 Compound of Formula I): The title compound was prepared according to General Synthetic Method D as described above, wherein in the compound of Formula II, R 2 =CH 3 , R 3 = H, G 1 =N(CH 3 ) 2 , n 2 =0,n 3 = 1, R 4b and R 5b =CH 3 , n 4 = 1, and Q 1 =CO 2 CH 3 1 H NMR (CDCl 3 , 200MHz) δ0.90(d, 3H, J=6.6Hz), 1.35(s, 6H), 2.21(s, 6H), 3.55-3.63(m, 1H), 3.70(s, 3H), 4.07(s , 2H), 5.09(d, 1H, J=9.8Hz), 7.01(d, 2H, J=9.6Hz), 7.10(s, 1H), 7.15(d, 1H, J=2.6Hz), 7.40(dd , 1H, J=1.4Hz, 8.6Hz), 7.67-7.71 (m, 4H); MS (ES) 410.0 (M+1).
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com