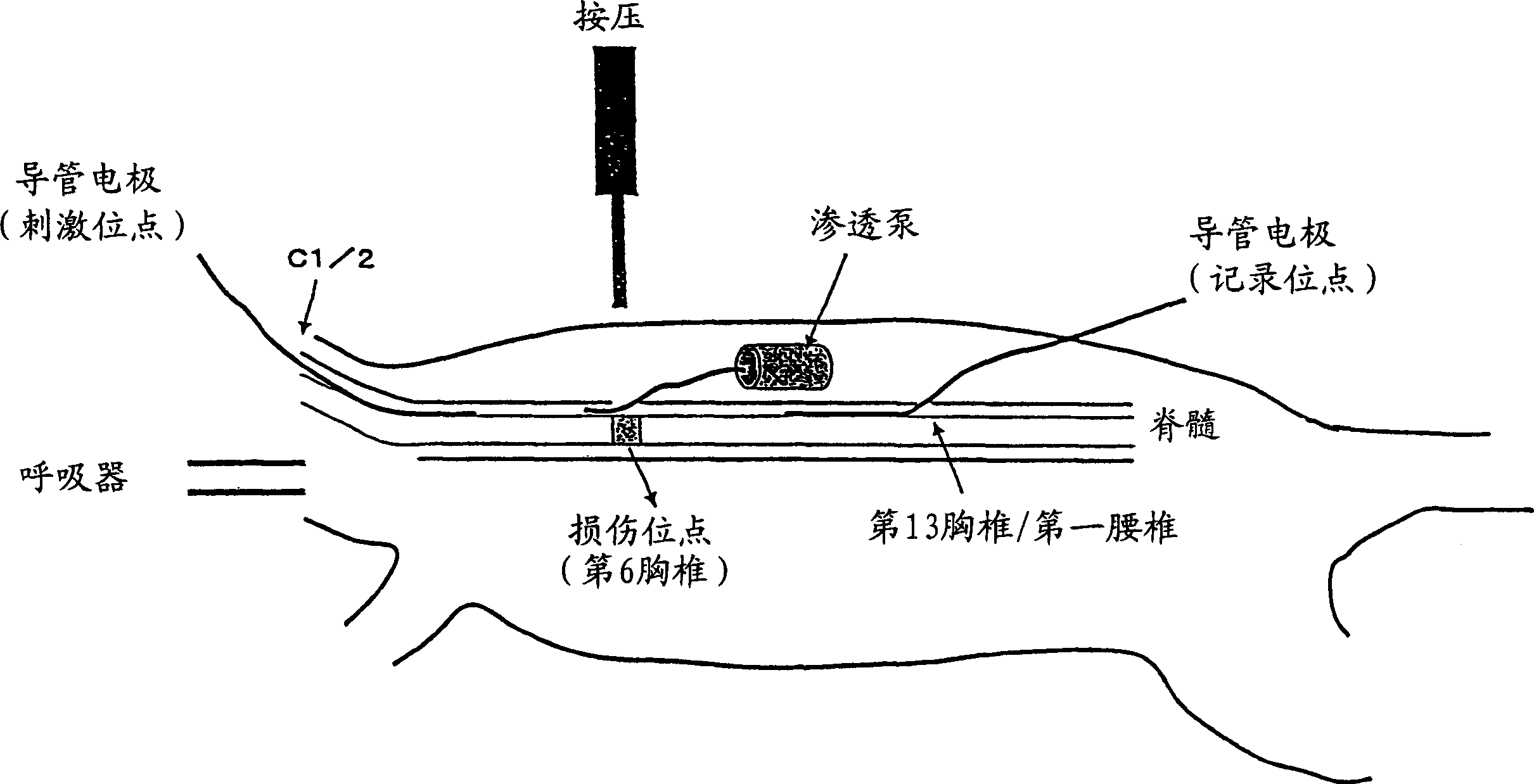
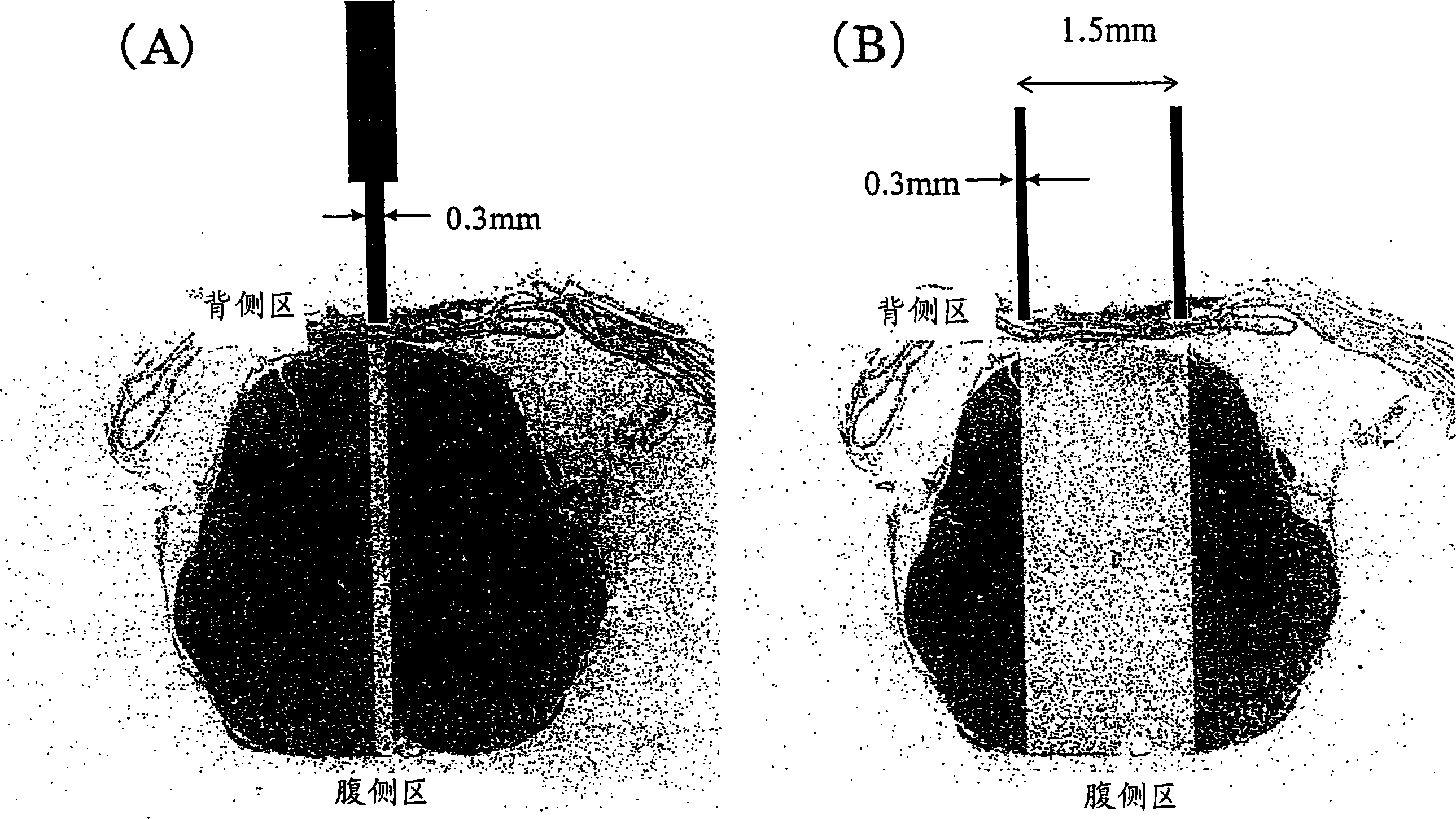
Remedy for nerve damage
A technique for nerve damage and therapeutic agents, applied in the field of therapeutic agents for nerve damage, capable of solving problems such as non-disclosure or prompting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0071] Embodiments of the present invention will be specifically described below. However, the scope of the present invention is not limited thereto.
[0072]
[0073] First, substances and the like used in these examples will be described.
[0074] Reagents, etc.
[0075] Low-molecular-weight HA is used as a low-molecular-weight saccharide composed of at least GlcA and / or GlcNAc.
[0076] HA from Seikagaku Corporation was used as the low molecular weight HA. The low molecular weight HA has the following structure and has the following characteristics (abbreviations used in this example are shown in brackets below. In the following formulae, the symbol "-" represents a glycosidic bond.).
[0077] Saturated HA tetrasaccharide (hereinafter referred to as "HA4").
[0078] GlcA-GlcNAc-GlcA-GlcNAc
[0079] According to the method described by Nagasawa et al. (Carbohyd. Res., 141, p99-110, 1985), HA4 was obtained by size fractionation of degradation products prepared by anion...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com