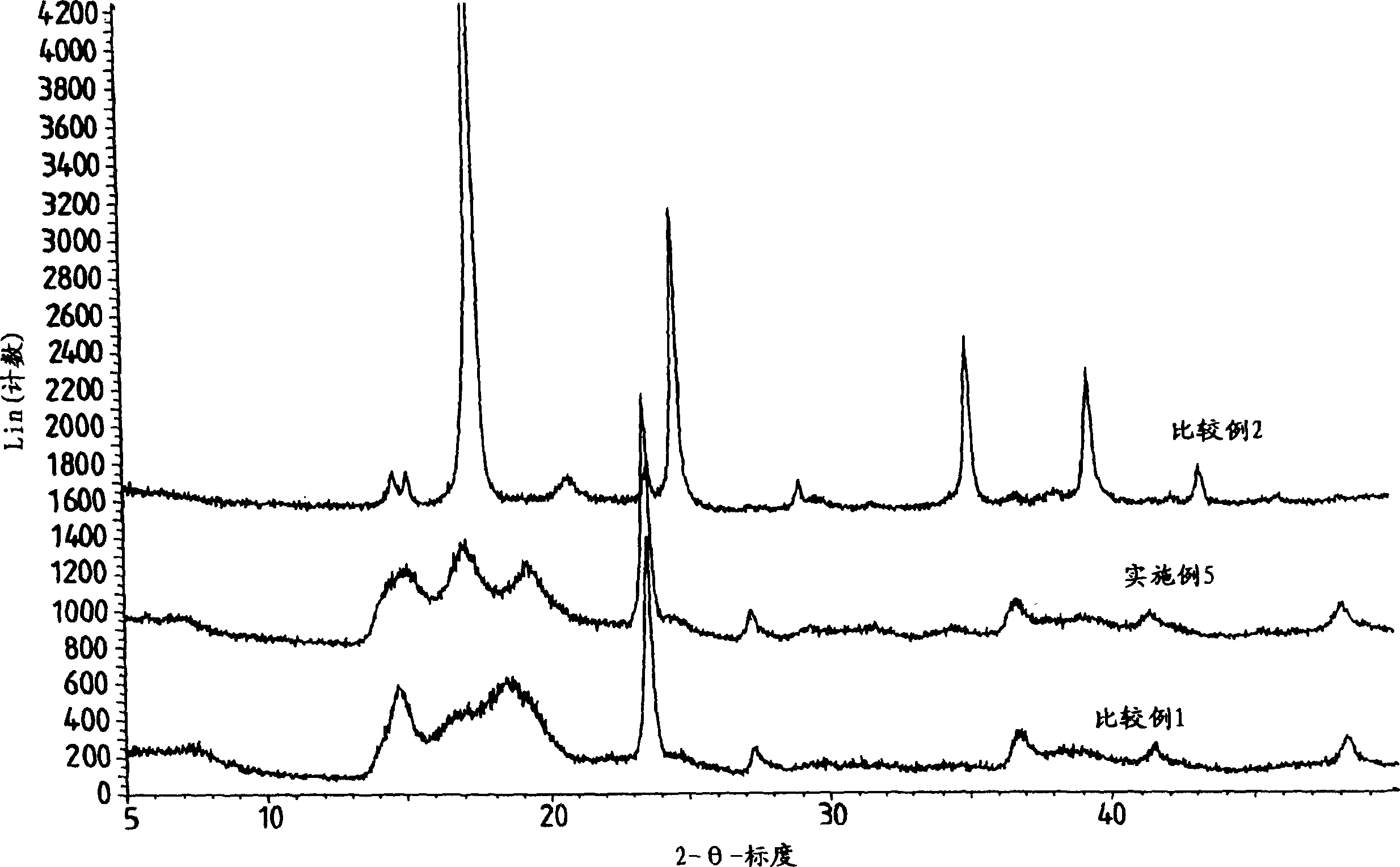
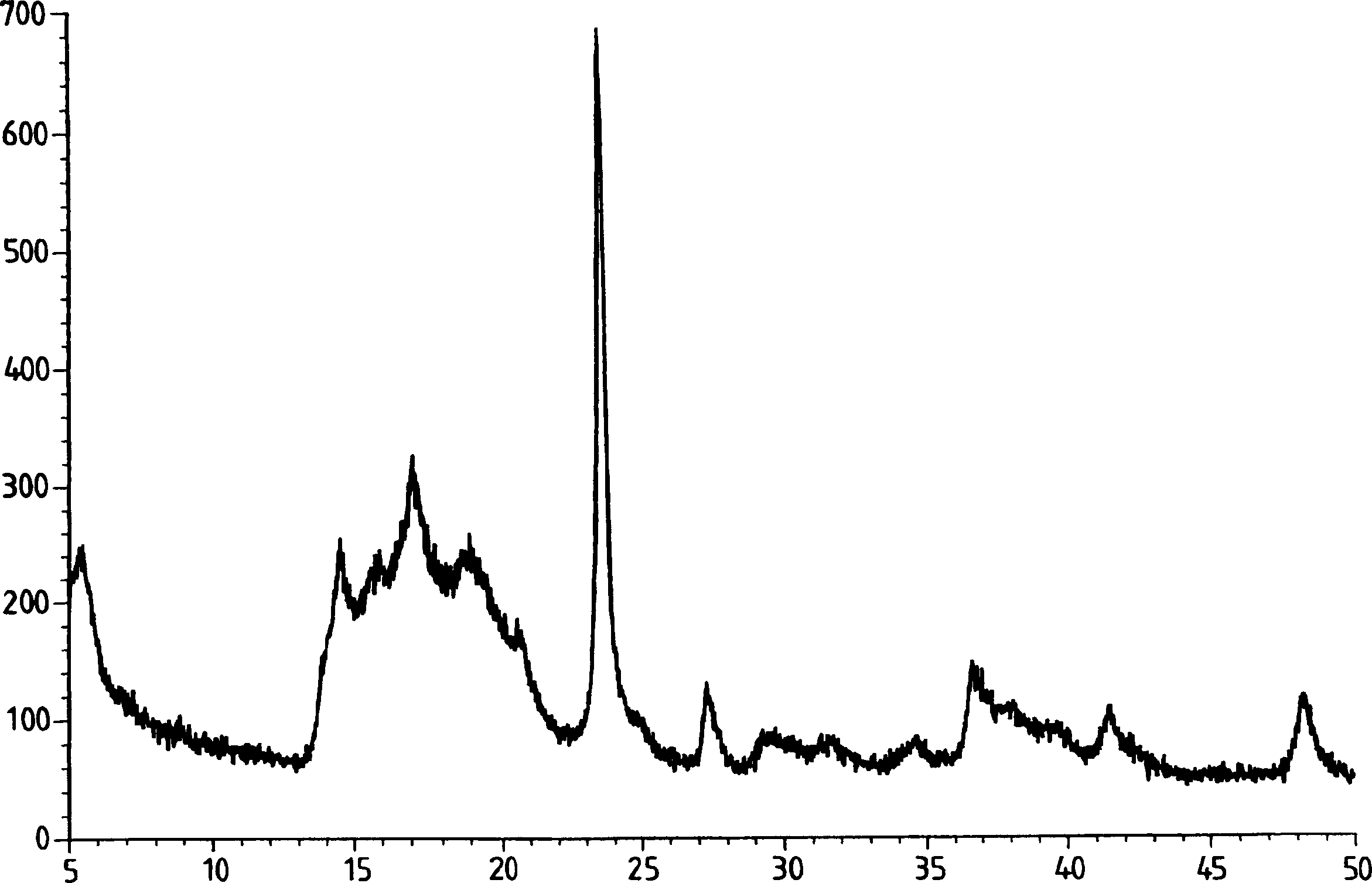
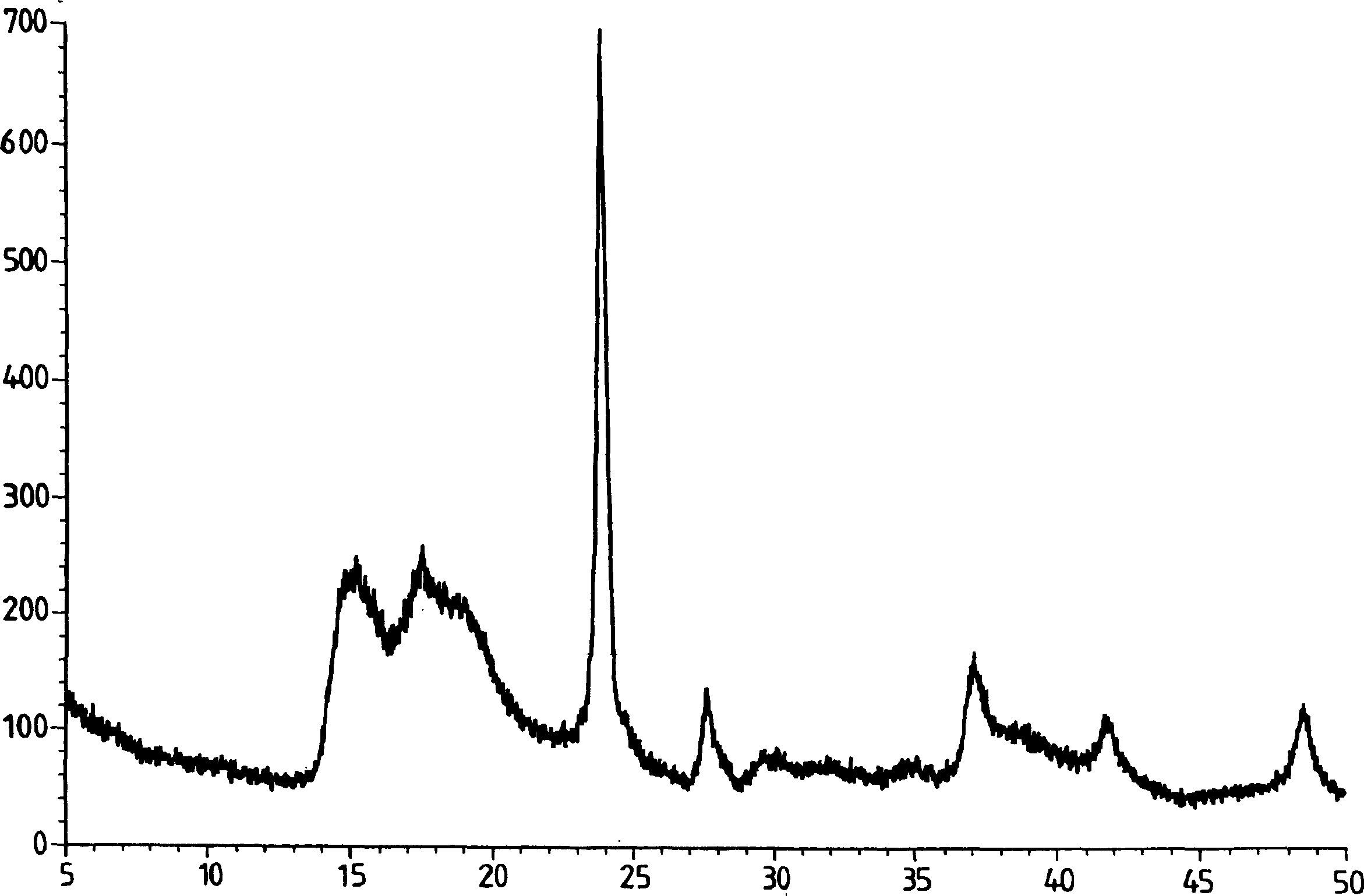
Process for preparation of double metal cyanide (DMC) catalyst
A double metal cyanide and catalyst technology, applied in the direction of metal cyanide, physical/chemical process catalysts, chemical instruments and methods, etc., can solve problems such as not providing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 3
[0091] DMC catalysts were prepared at a 2:2 Zn / Co molar ratio with addition of NaCl to maintain the molar ratio of metal salt anion to metal cyanide anion as follows: 1 L baffled round bottom flask equipped with mechanical paddle stirrer, heating mantle and thermometer . Deionized water (275 g) was added to the flask, followed by technical grade zinc chloride (6.07 g) and sodium chloride (60.0 g). Add enough zinc oxide to bring the system to an alkalinity of 6.48% ZnO. Tert-butanol (40.0 g) was then added and the solution was heated to 50°C with stirring at 400 rpm.
[0092] A second solution was prepared by dissolving potassium hexacyanocobaltate (7.4 g) in deionized water (100 g). The potassium hexacyanocobaltate solution was added to the zinc chloride solution over 1 hour. Stirring was continued for an additional 60 minutes at 50°C after the addition was complete. A third solution was prepared with 1000 Da diol (7.9 g), t-butanol (27.1 g) and water (14.9 g) and added to...
Embodiment 4
[0096] DMC catalysts were prepared at a Zn / Co molar ratio of 2.0 and NaCl was added to maintain the molar ratio of metal salt anion to metal cyanide anion as follows: A 1 L baffled round bottom flask was equipped with a mechanical paddle stirrer, heating mantle and thermometer. Distilled water (275 g) was added to the flask, followed by technical grade zinc chloride (5.73 g) and sodium chloride (60.0 g). Add enough zinc oxide to make the system alkalinity reach 3.73% ZnO. Tert-butanol (40.0 g) was then added and the solution was heated to 50°C with stirring at 400 rpm.
[0097] Potassium hexacyanocobaltate (7.4 g) was dissolved in distilled water (100 g) to prepare a second solution. The potassium hexacyanocobaltate solution was added to the zinc chloride solution over 1 hour. Stirring was continued for an additional 60 minutes at 50°C after the addition was complete. A third solution was prepared with 1000 Da diol (7.9 g), t-butanol (27.1 g) and water (14.9 g) and added to...
Embodiment 5
[0101] DMC catalysts were prepared at a Zn / Co molar ratio of 1.5 and NaCl was added to maintain the molar ratio of metal salt anion to metal cyanide anion as follows: A 1 L baffled round bottom flask was equipped with a mechanical paddle stirrer, heating mantle and thermometer. Distilled water (275 g) was added to the flask, followed by technical grade zinc chloride (4.22 g) and sodium chloride (60.0 g). Add enough zinc oxide to make the system alkalinity reach 4.88% ZnO. Tert-butanol (40.0 g) was then added and the solution was heated to 50°C with stirring at 400 rpm.
[0102] Potassium hexacyanocobaltate (7.4 g) was dissolved in distilled water (100 g) to prepare a second solution. The potassium hexacyanocobaltate solution was added to the zinc chloride solution over 1 hour. Stirring was continued for an additional 60 minutes at 50°C after the addition was complete. A third solution was prepared with 1000 Da diol (7.9 g), t-butanol (27.1 g) and water (14.9 g) and added to...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com