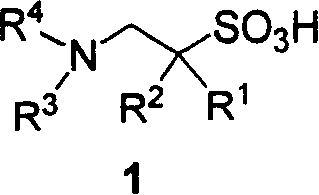
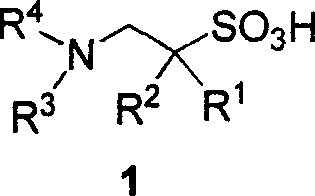
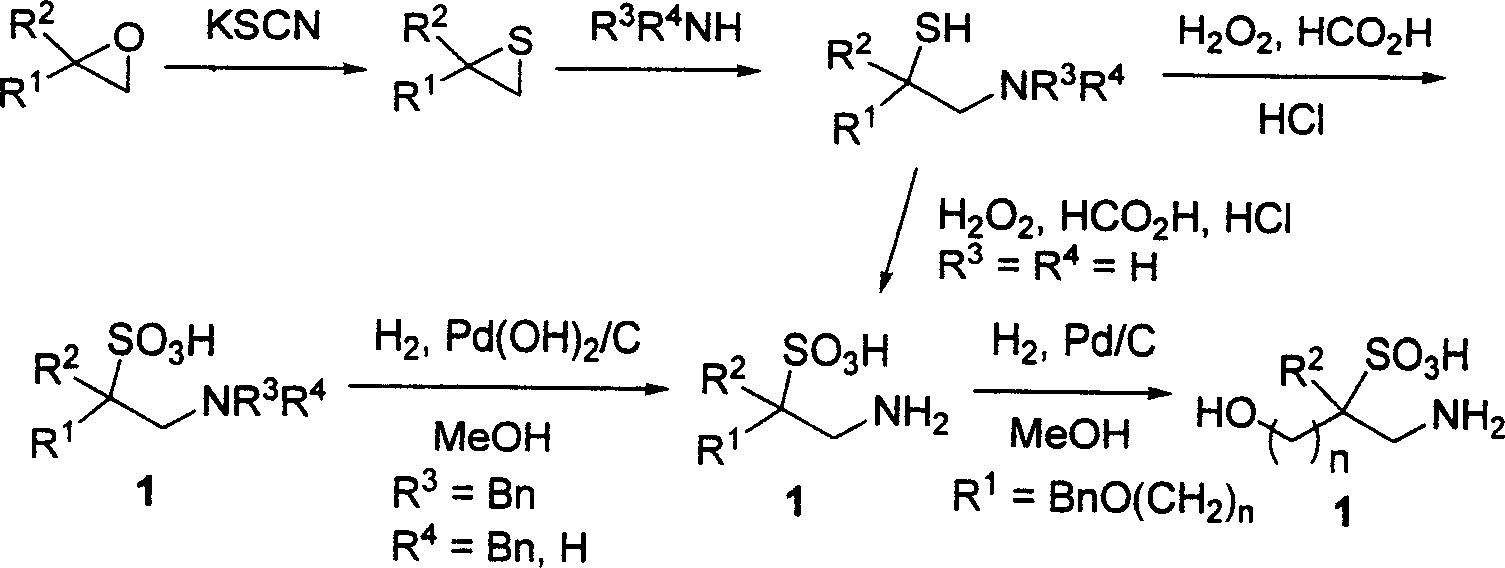
Derivative of substitutional taurine and preparation method
A taurine derivative, selected technology, applied to the preparation of sulfonic acid, preparation of organic compounds, chemical instruments and methods, etc., to achieve the effect of easy preparation and easy access to raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Preparation of 1-N, N-diethylamino-3-phenoxypropane-2-sulfonic acid (1a)
[0058] (1) ring vulcanization:
[0059] Add 5.0g (33mmol) of 2-phenoxymethyloxirane, 34g (175mmol) of potassium thiocyanate-silica gel mixture and 100mL toluene into a 250mL three-necked flask, stir, and maintain a reflux state. TLC monitoring (petroleum ether-ethyl acetate 3:1, v / v, silica gel plate, ethylene oxide R f =0.50, sulfide R f =0.67), after 5h, the raw material point disappeared, and the reaction was stopped. The solid was filtered off with a quartz sand funnel, and the solvent was spun off at 60° C. to obtain 5.3 g of 2-phenoxymethylthioethane as a light yellow liquid, with a yield of 96 %. The yield without the catalyzed potassium thiocyanide-silica gel mixture was 86%.
[0060] (2) Amine ring opening:
[0061] Add a solution of diethylamine (6.57g, 90mmol) in 100mL of benzene-ethanol (the general ratio is 1:9 to 9:1) into a 250mL round bottom flask, and control the temperature...
Embodiment 2
[0065] Preparation of 1-N, N-dibenzylamino-3-phenoxypropane-2-sulfonic acid (1b)
[0066] According to the method described in Example 1, dibenzylamine was used to open the ring to obtain 1-N, N-dibenzylamino-3-phenoxy-2-propanethiol, a colorless liquid, and the yield was 75%. 1 H NMR (200MHz, CDCl 3 )δ7.45-6.80 (m, 15H, ArH), 4.08 (dd, J=5.2, 9.5Hz, 1H in OCH 2 ), 3.91 (dd, J=6.2, 9.5Hz, 1H in OCH 2 ), 3.62(s, 4H, 2NCH 2 ), 3.45 (dddd, J=5.2, 6.2, 7.2, 7.6Hz, 1H, SCH), 2.86 (ddd, J=7.6, 13.2Hz, 1H in NCH 2 ), 2.60 (dd, J=7.2, 13.2Hz, 1H in NCH 2 ), 1.59 (s, br, 1H, SH). 13 C NMR (50MHz, CDCl 3 )δ158.5, 138.9, 129.5, 129.1, 128.3, 127.2, 121.0, 114.6, 70.8, 58.8, 57.6, 37.9.
[0067] Starting from 1-N,N-dibenzylamino-3-phenoxy-2-propanethiol, the oxidation method described in Example 1 was used to prepare 1-N,N-dibenzylamino-3-phenoxy propane-2-sulfonic acid. Colorless crystals, mp 199-201°C, yield 81%. 1 H NMR (200MHz, CDCl 3 )δ10.34 (s, br, 1H, SO 3 H), 7.62-6.71...
Embodiment 3
[0069] (S)-1-N, Preparation of N-dibenzylamino-3-phenoxypropane-2-sulfonic acid (1c)
[0070] According to the method described in Example 2, (S)-2-phenoxymethyl oxirane is used as raw material to obtain (R)-2-phenoxymethyl sulfide, and dibenzylamine ring-opening obtains ( S)-1-N, N-dibenzylamino-3-phenoxy-2-propanethiol, colorless liquid, yield 78%.[α] 20 D =+4.2(c 1.10, CHCl 3 ). 1 H NMR (200MHz, CDCl 3 )δ7.38-6.78 (m, 15H, ArH), 4.07 (dd, J=4.8, 9.2Hz, 1H in OCH 2 ), 3.91 (dd, J=3.2, 9.2Hz, 1H in OCH 2 ), 3.61 (s, 4H, 2NCH 2 ), 3.41 (dddd, J=3.2, 4.8, 7.2, 7.6Hz, 1H, SCH), 2.85 (dd, J=7.6, 13.0Hz, 1H in NCH 2 ), 2.59 (dd, J=7.2, 13.0Hz, 1H in NCH 2 ), 2.02(s, br, 1H, SH). 13 C NMR (50MHz, CDCl 3 )δ158.5, 138.9, 129.4, 129.0, 128.3, 127.2, 121.0, 114.6, 70.7, 58.8, 57.6, 37.9.
[0071] According to the method described in Example 1, (S)-1-N, N-dibenzylamino-3-phenoxy-2-propanethiol was oxidized to obtain (S)-1-N, N-dibenzyl Amino-3-phenoxypropane-2-sulfonic acid....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com