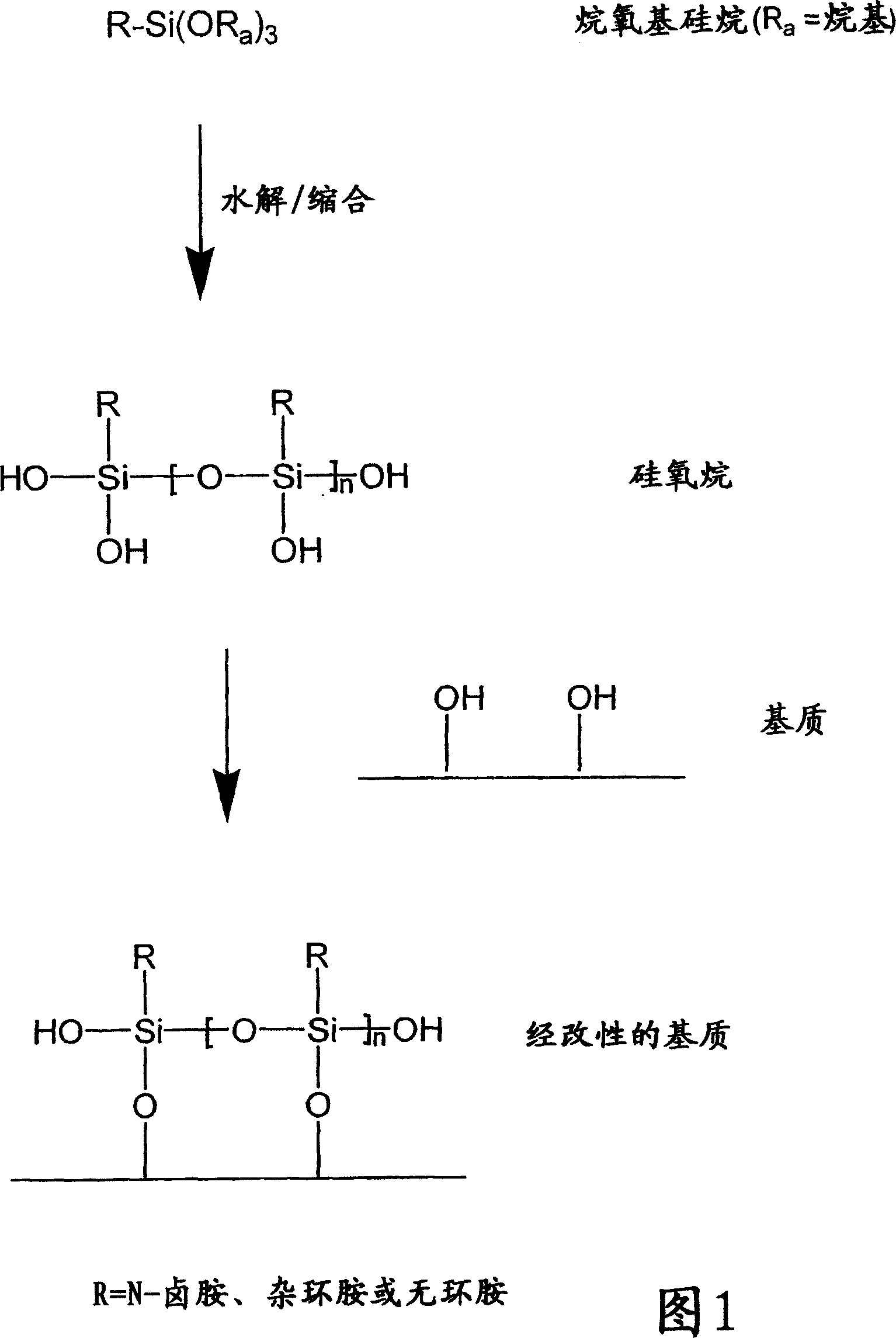
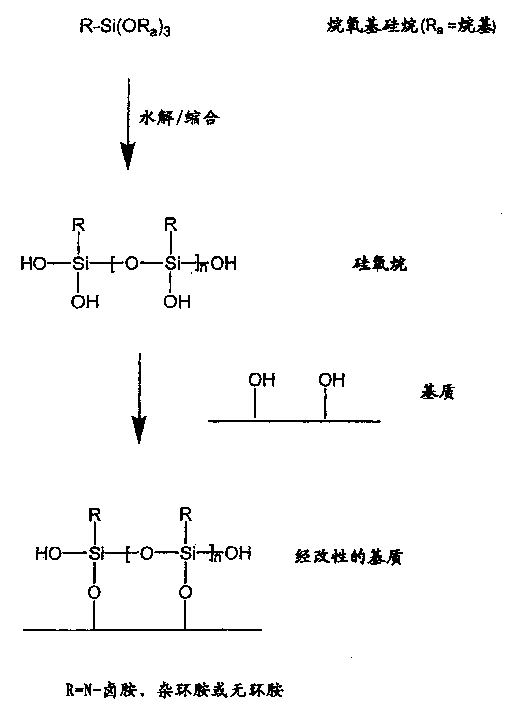
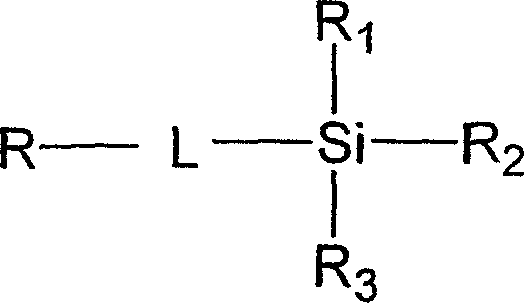N-halamine siloxanes for use in biocidal coatings and materials
一种烷基、化合物的技术,应用在用于杀生物涂层和材料的杂环卤胺硅氧烷领域,能够解决接触时间长等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Preparation of representative unhalogenated silane compounds
[0110] Two trialkoxysilylpropylhydantoin derivatives were prepared according to methods similar to those outlined in US Patent 4,412,078.
[0111]Equip a 1-liter three-necked round-bottom flask with a condenser, dropping funnel, and thermometer. A mixture of 500 mL of ethanol, 64.0 g (0.5 mol) of 5,5-dimethylhydantoin (Acros, Inc) and 28.0 g (0.5 mol) of potassium hydroxide was added to the flask. The mixture was heated to boiling point until the solution became clear. The solid potassium salt of 5,5-dimethylhydantoin was then isolated by evaporating the ethanol solvent and the water produced in the reaction under reduced pressure. The salt was dried under vacuum at 60°C for 4 days to form the anhydrous potassium salt. The dried salt was then returned to the one liter flask where it was mixed with 500 mL of anhydrous N,N-dimethylformamide (DMF) and the mixture was heated at 60° C. until a c...
Embodiment 2
[0114] Preparation and biocidal efficacy of representative chlorosilane compounds
[0115] A portion (6.11 g, 0.021 mol) of 3-trimethoxysilylpropylhydantoin prepared as described in Example 1 was dissolved in 30 mL of dichloromethane in a 125 mL Erlenmeyer flask. Then 2.30 g (0.021 mol) of tert-butyl hypochlorite prepared according to the method of Mintz et al. 3 hours and exclude all light. The tert-butanol produced in the reaction was removed by evaporation in vacuo. The product 1-chloro-3-trimethoxysilylpropyl-5,5-dimethylhydantoin was produced as a yellow oil in 89.7% yield. It was stored at 4 °C in the absence of light until use. The total chlorine content was determined by iodometry / thiosulfate titration to be 10.36%, whereas the theoretically possible value is 10.94%. 3-trimethoxysilylpropylhydantoin at δ7.07 1 The H NMR signal disappeared upon chlorination, indicating the presence of chlorine at the 1-position of the hydantoin moiety.
[0116] A 100.8 mg / L ...
Embodiment 3
[0118] Preparation of biocidal paper
[0119] Cut a handful of white and tan business office envelopes into small squares. A 2% aqueous base solution of 3-triethoxysilylpropyl-5,5-dimethylhydantoin (pH 3 due to NaOH addition) prepared as described in Example 1 was dissolved in a nebulizer bottle Spray onto both sides of the paper samples until they become saturated. The wet samples were then cured until dry at 60 °C for 15 min. Both sides of the cured sample were then sprayed with 10% CLOROX bleach until saturated, allowed to stand at room temperature for 10 minutes, rinsed 5 times with 50 mL of partially chlorine-free water, and dried at room temperature. Samples were stored in a vacuum desiccator until analysis and microbiological characterization.
[0120] Iodometric / thiosulfate titration was used to determine the chlorine loading on squares of both papers as a function of time after chlorination. The data are shown in Table 1.
[0121] sa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com