Phosphorescent compounds and devices comprising the same
A compound and independent technology, applied in the direction of osmium organic compounds, ruthenium organic compounds, indium organic compounds, etc., can solve the problems of outdated cathode ray tubes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0206] Embodiment 1: compound and element performance
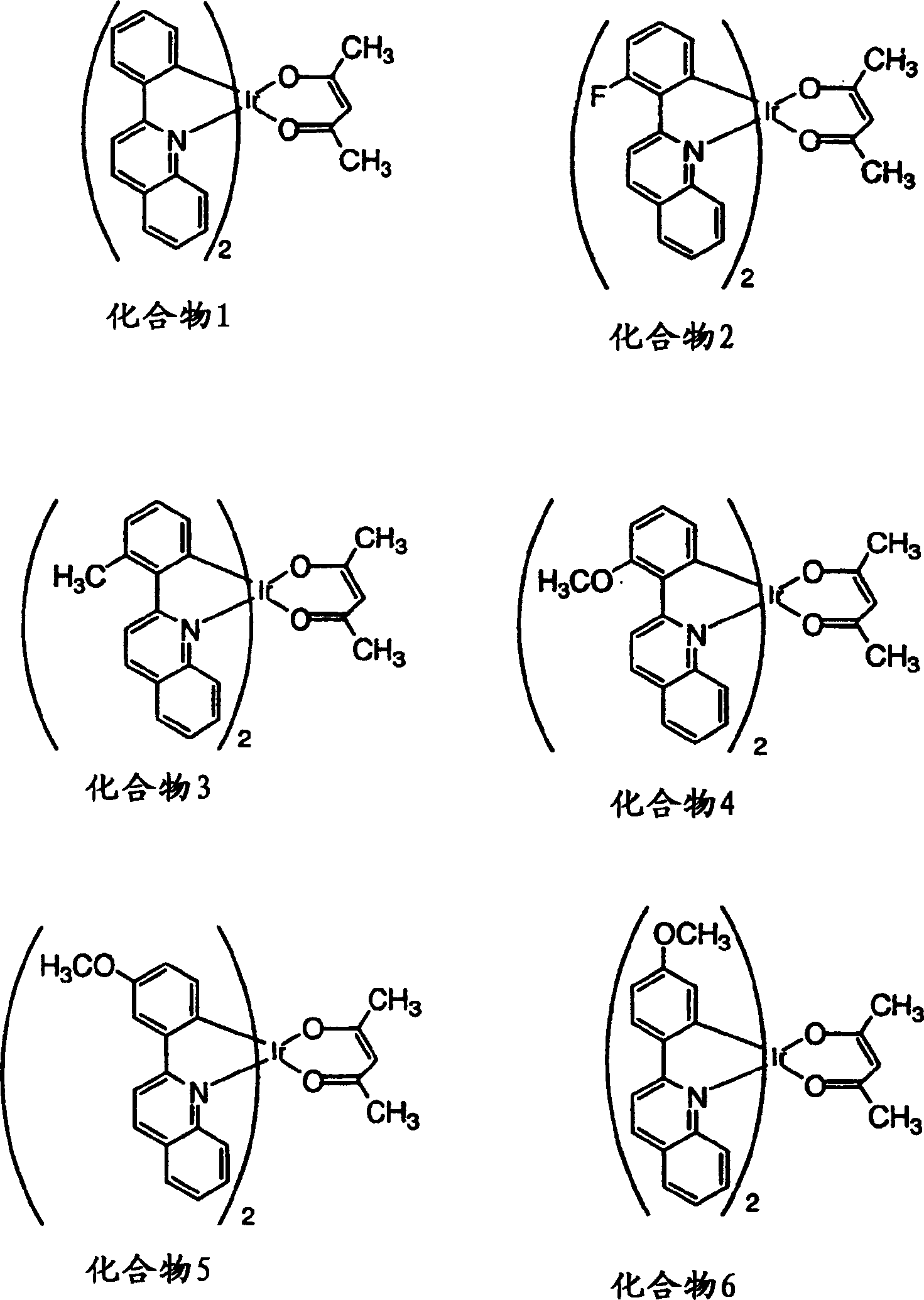
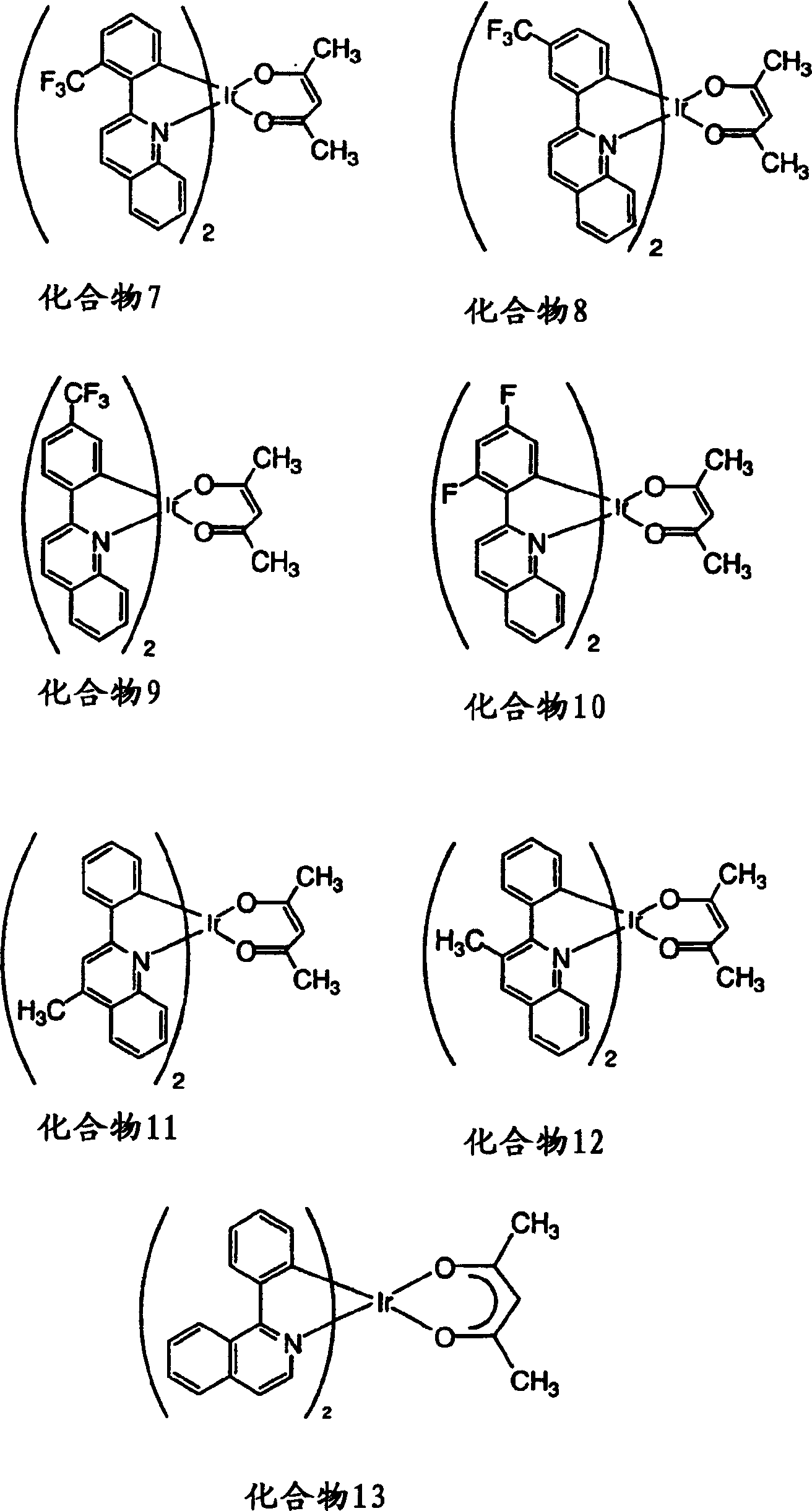
[0207] Characterization of substituted phenylquinolyl iridium(III) acetylacetonates 2-13 and comparative compound 1 (see figure 1 and 2 ) and as emissive dopant for glass / ITO / CuPc / NPD / CPB: dopant / BAlq / Alq 3 / LiF / Al structure of the organic light-emitting device. The photophysical properties of the compounds and components are surprisingly excellent, as shown in Table 1. The element glow extends from orange to deep red. The highest efficiencies were obtained with orange-red elements including compound 11. Typically, the brightness level of a color display, i.e., 10-1000cd / m 2 The external quantum efficiency greater than 8% and the luminous efficiency higher than 20cd / A are obtained. These efficiencies are almost better than known red-emitting fluorescent elements, such as Hatwar et al. in Proceedings of the10 th The DCJTB element reported by International Workshop of Inorganic and Organic Electroluminescence, Decem...
Embodiment 2
[0209] Example 2: Change the luminous color
[0210] By attaching the activating substituent to the coordinating atom associated with the HOMO of the compound of the invention, a red shift of 50 nm in the photoluminescence maximum relative to the unsubstituted reference compound was observed. For example, the photoluminescence maximum of compound 5 (see figure 1 ) reported in Table 1 as 656 nm, which is an increase of 50 nm relative to the reference compound with a photoluminescence maximum at 606 nm (Table 1) (see figure 1 ). MO Computational Oracle, R 3 Substituents contribute to the HOMO (see Figure 7 ). In fact, the activated methoxyl substituting the R 3 bit on, with where R 3 Compared with the reference compound 1 which is hydrogen, there is an obvious red shift in the photoluminescence spectrum.
Embodiment 3
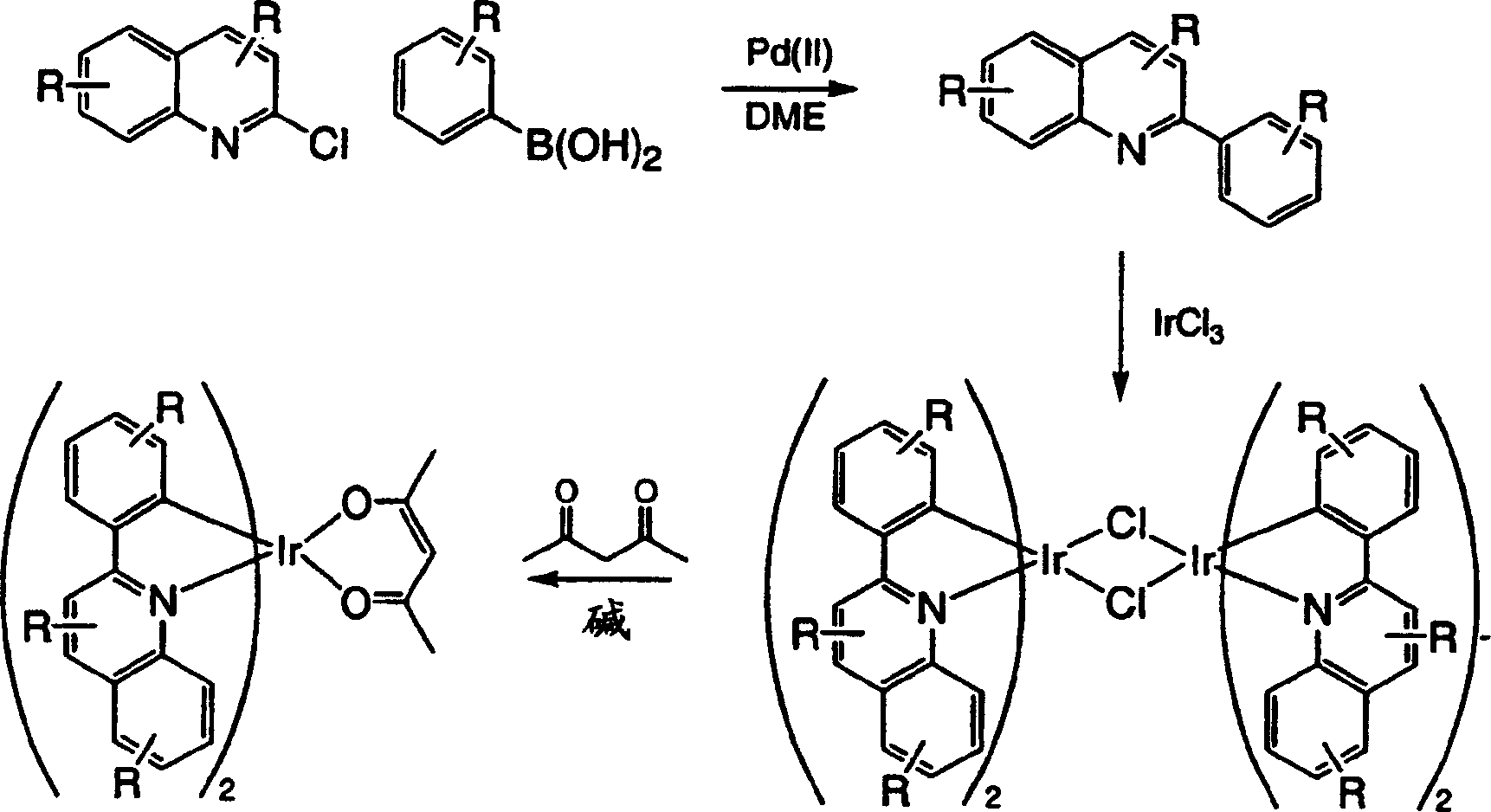
[0211] Embodiment 3: the synthesis of two (2-phenylquinoline) iridium (III) acetylacetonate (compound 1)
[0212] step 1
[0213] 2-Phenylquinoline (6.0 g, 29 mmol) and iridium(III) chloride hydrate (5.2 g, 14 mmol) were added to a flask containing 80 mL of 2-methoxyethanol and 20 mL of distilled water . The reaction mixture was heated to reflux and stirred under a nitrogen atmosphere for 24 hours. After cooling, the red precipitate formed was vacuum filtered and washed first with absolute ethanol and then with hexane. The dichloro-bridged dimer was dried in a vacuum oven to afford 6.7 g of product (38% yield). The product was used directly in the next step without any further purification.
[0214] Step 2:
[0215] The dichloro-bridged dimer (6.7 g, 5.3 mmol) was added to a solution containing 2-methoxyethanol (150 mL) in 200 mL. Sodium carbonate (5.6 g, 53 mmol) and 2,4-pentanedione (5.3 g, 53 mmol) were added to the reaction mixture. The reaction mixture was heated t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| external quantum efficiency | aaaaa | aaaaa |
| external quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com