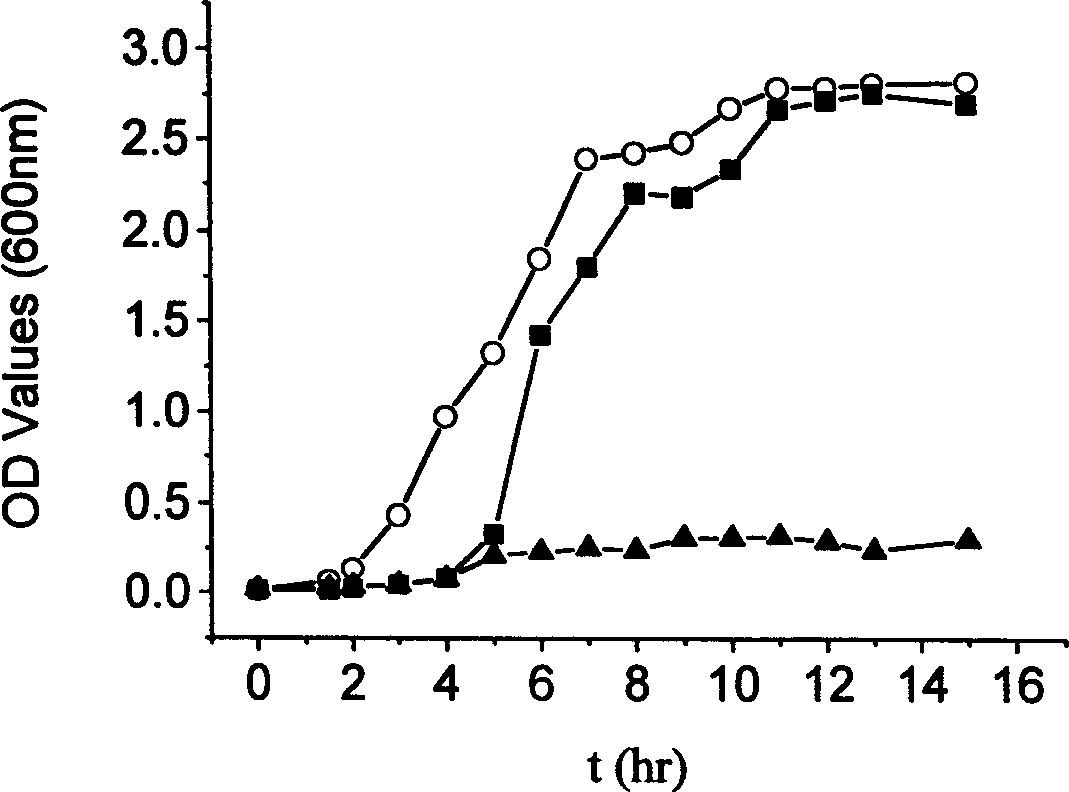
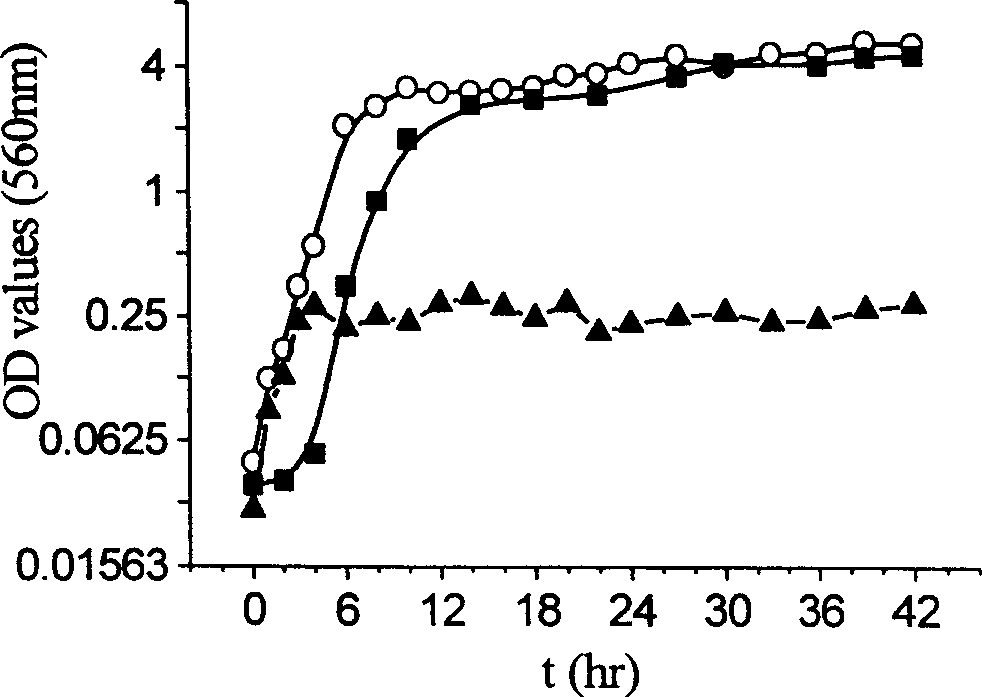
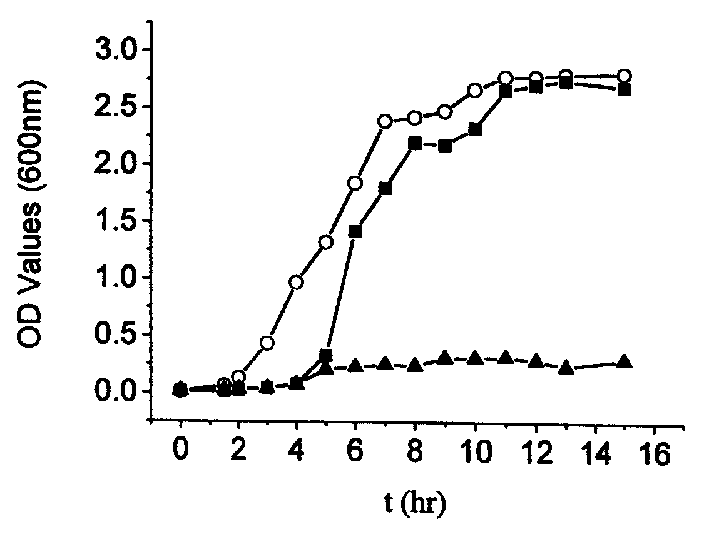
Prepn of biocompatible SA/CS-CaCl2/PMCG microcapsule
A biocompatibility and microcapsule technology, applied in biochemical equipment and methods, microcapsule preparation, microsphere preparation, etc., can solve the problem of inability to culture microcapsule cells, composition of impure components, and preparation of no reports Process and other issues, to achieve the effect of easy application and promotion, cheap raw materials, and good biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0026] The materials used for the preparation of novel biological microcapsules of the present invention are as follows:
[0027] 1) Sodium cellulose sulfate (CS), prepared by the Institute of Bioengineering, Zhejiang University.
[0028] 2) Sodium alginate (SA), purchased from Shanghai Institute of Biochemistry.
[0029] 3) Polymethylenebiguanidine·hydrogen chloride (PMCG), which was purchased from Scientific Polymer Products, USA, and other reagents such as calcium chloride were commercially available analytical reagents.
[0030] Different examples are given below to illustrate the present invention:
Embodiment 1
[0032]1) At room temperature, fully mix absolute ethanol and PMCG aqueous solution at a ratio of 2:1, let stand for 30 minutes, centrifuge the mixture at 6000rpm for 15min, collect the precipitate, wash it with absolute ethanol for 3 times, and then wash the precipitate The product was freeze-dried to remove residual ethanol and other small molecular impurities. Finally, the precipitate was dried in a vacuum oven at 50° C. for 48 hours and set aside;
[0033] 2) Fully mix 2% sodium alginate (SA) and 3.5% cellulose sodium sulfate (CS), the ratio of the two is 1.5, this mixture is the polyanion solution of this system, pass the polyanion solution through the syringe Drop in 3% calcium chloride solution and solidify, and solidification time is 15 minutes, obtains solid pre-capsule, washes pre-capsule with deionized water, then it is changed into 2% polymethylene biguanidine after the purification. Reaction in hydrogen chloride (PMCG) solution, the reaction time is 15 minutes, th...
Embodiment 2
[0036] 1) Fully mix absolute ethanol and PMCG aqueous solution at a ratio of 0.5:1, let stand for 25 minutes, centrifuge the mixture at 4000 rpm for 5 minutes, collect the precipitate, wash it twice with the corresponding organic solvent, and then freeze the precipitate Dry to remove residual organic solvents and other small molecular impurities, and finally dry the precipitate in a vacuum oven at 37°C for 24 hours to obtain polymethylenebiguanidine·hydrogen chloride (PMCG) from which small molecular impurities are removed;
[0037] 2) Fully mix 0.5% sodium alginate (SA) and 0.5% cellulose sodium sulfate (CS), the ratio of the two is 0.2, this mixture is the polyanion solution of this system, pass the polyanion solution through the syringe Drop into 0.5% calcium chloride solution to solidify, and the solidification time is 5 minutes to obtain a solid pre-capsule, wash the solid pre-capsule with deionized water, and then transfer it to 1% polymethylene bismuth to remove small mo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com