Human pancreatic epithelial progenitor cells and methods of isolation and use thereof
A technology of progenitor cells and islet cells, applied in the fields of developmental biology and cell biology, can solve problems such as inability to accurately reflect the physiological parameters of pancreatic cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Embodiment 1
[0075] Examples Example 1: Isolation of pancreatic progenitor cells
[0076] Fetal pancreas (gestational age 14-22 weeks) was dissected mechanically by microdissection under a stereomicroscope, followed by enzymatic dissociation. Enzyme treatment included placing partially dissociated tissues in 1 ml of F12 / DMEM medium containing 5 mg / ml collagenase-dispase, 20 μg / ml soybean trypsin inhibitor, and 50 μg / ml DNase, and incubated at 37°C for 15 minute.
[0077] insulin
[0078] Dispense the resuspended cell aggregates into fibronectin-coated wells (6-12) of a 24-well plate and store in a humidified 37 °C, 5% CO 2 Incubate for 72 hours in an incubator. After 72 hours, epithelial cells formed suspended spherical structures ( figure 1 A), while mesenchymal or stromal cells adhere to the well walls. When a monolayer structure is desired, collect 6 wells of pancreatic aggregates or spheres with a micropipette and place in a collagen-coated 60 mm Pe...
Embodiment 2
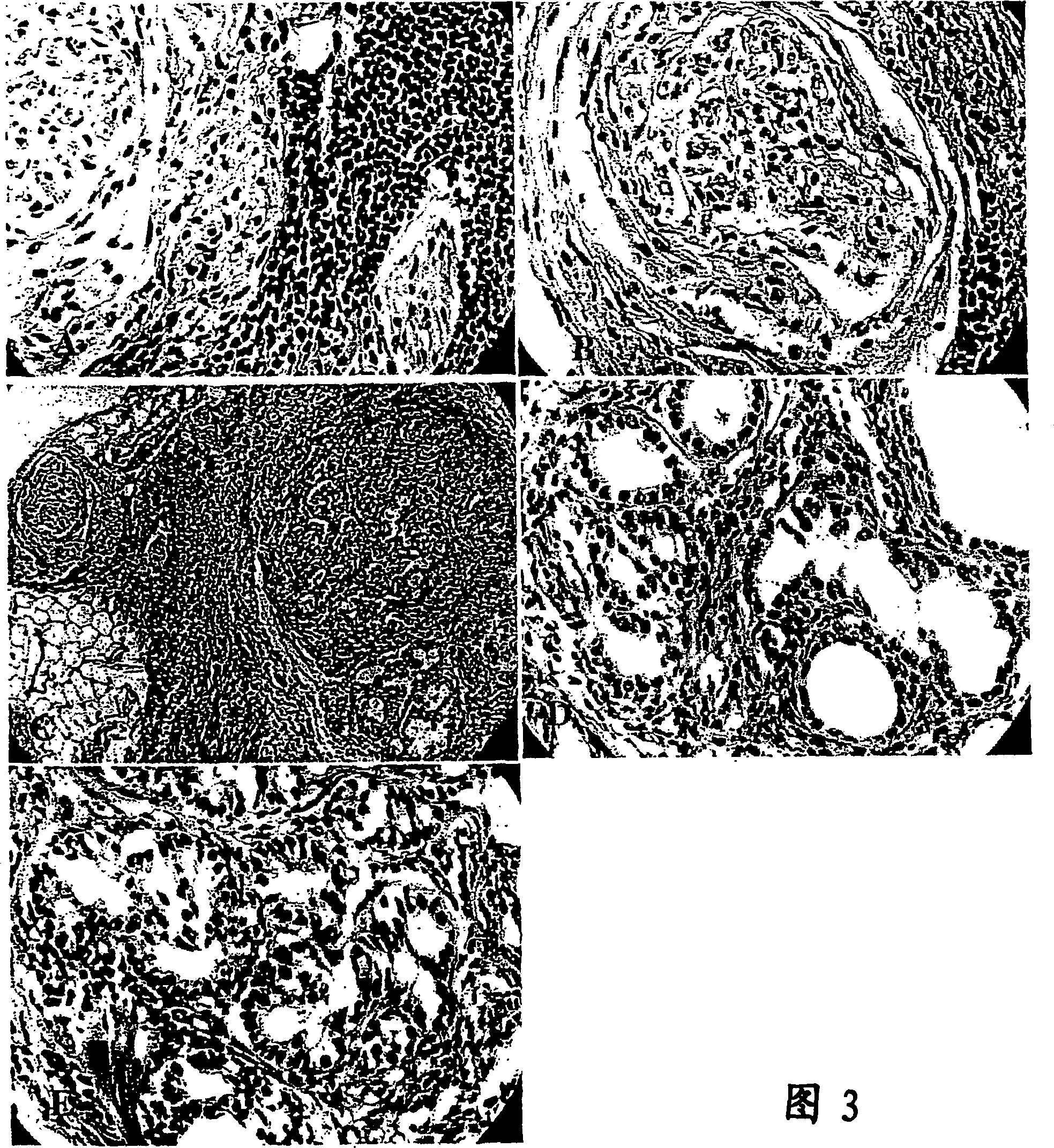
[0078] Dispense the resuspended cell aggregates into fibronectin-coated wells (6-12) of a 24-well plate and store in a humidified 37 °C, 5% CO 2 Incubate for 72 hours in an incubator. After 72 hours, epithelial cells formed suspended spherical structures ( figure 1 A), while mesenchymal or stromal cells adhere to the well walls. When a monolayer structure is desired, collect 6 wells of pancreatic aggregates or spheres with a micropipette and place in a collagen-coated 60 mm Petri dish using F12 / DMEM as the basic nutrient medium and supplemented with this article Disclosed Nutrients. Within 24 hours, the above-mentioned structures adhered and cells from the above-mentioned structures spread out on the collagen to form an epithelial monolayer ( figure 1 B). These pancreatic progenitor cells can be passaged at least three times ( figure 2 ). Example 2: Use of pancreatic progenitor cells in transplantation
[0079] For the purpose of recombinant transplantation, cells were...
Embodiment 3
[0083] After transplanting pancreatic spheres into the subrenal membrane of mice and maintaining them at the site for 6-8 weeks, the grafts were harvested and analyzed to identify pancreatic cells by immunohistochemistry and function. showed that the grafts expressed insulin and glucagon ( Figure 4 , 5 , and 7). In addition, tissue grafted recombinants were shown to form ductal structures ( Figure 6 ). Thus, the tissue-reconstituted graft produces functional pancreatic cells capable of expressing insulin and glucagon and forming ductal structures.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com