APRIL receptor (BCMA) and uses thereof
A variant, amino acid technology, applied in the direction of cytokine/lymphokine/interferon receptor, receptor/cell surface antigen/cell surface determinant, antibody, etc., can solve the problem of little curative effect and multiple existing treatments Insufficient types of tumors and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] Embodiment 1: Detect the combination of APRIL and APRIL-R with plate test
[0116] In this example, the binding of BCMA to April was tested.
[0117] To test whether BCMA binds April, we performed a co-immunoprecipitation assay. Two soluble proteins, hBCMA-Fc and myc-mApril, were used in this assay.
[0118] HBCMA-Fc and LTbR-Fc and the following different TNF ligands were added to medium containing 10% FBS: myc-mApril; myc-CD40L and myc-RANKL and incubated for 1 / 2 hour at room temperature. Fc protein was combined with protein A beads for 1-2 hours, washed 3 times with 1ml PBS, immunoblot analysis was performed with mouse monoclonal antibody 9E10 (anti-myc), and color development was performed with enhanced chemiluminescence.
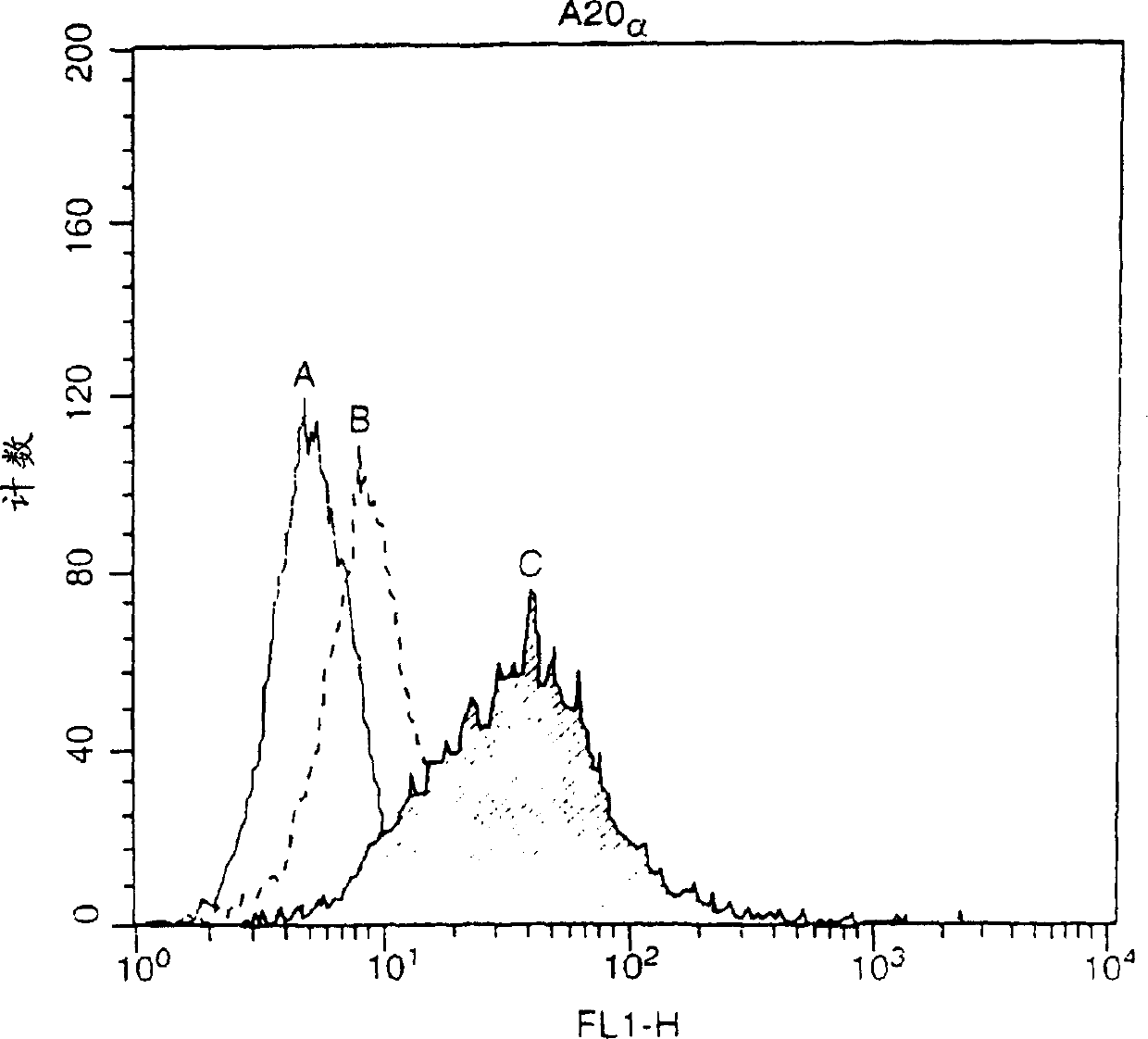
[0119] The detection of myc-APRIL in hBCMA-Fc immunoprecipitate showed that the interaction between BCMA and April was specific because other TNF ligands myc-CD40L and myc-RANKL could not bind to BCMA. Myc-April does not bind LTbR-Fc.
[0120...
Embodiment 2
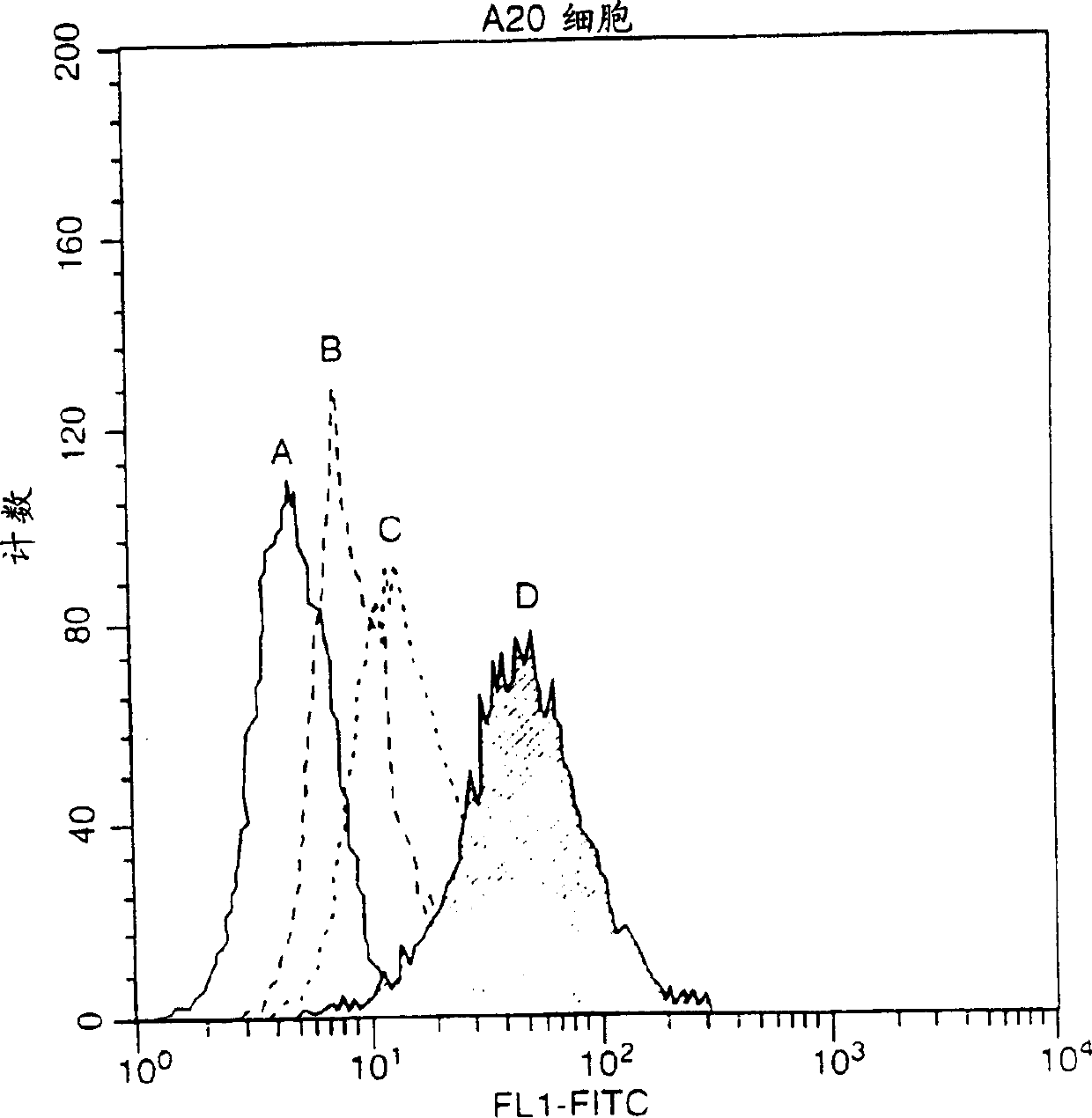
[0122] This example describes the interaction between hBCMA-Fc and FLAG-hAPRIL.
[0123] ELISA test: Coat the plate with pH 9.6 carbonate solution (1 ug / ml) of receptor-Fc fusion protein (hBCMA-Fc739 or hTNFR2-Fc-492), overnight at 4°C. Block with PBS / 5% skim milk powder / 0.5% Tween-20 for 2 hours at room temperature. Use 100 μl blocking solution to make 2-fold serial dilutions of the ligand (TNFa-197 starts from 1000ng / ml, muAPRIL-657 starts from 1000ng / ml, hApril-507 starts from 2000ng / ml (inactive), hApril-429 starts from 5x concentrated culture medium starts). After incubation with ligand, the plates were washed with PBS containing 0.5% Tween-20 and then hybridized with anti-FLAG mAb M2 in dilution buffer at a concentration of 0.5 μg / ml. The antibody was then detected with anti-mouse-PO 1 / 2000 and enzymatic color development (OPD).
[0124] Immunoprecipitation assay: 293T cells were transfected with the indicated expression plasmids (Rec-Fc or flag ligand) in a 9cm plate...
Embodiment 3
[0126]This example describes the binding of myc-mAPRIL, hKayL-440(hAPRIL) and Flag-mAPRIL to hBCMA-Ig, hLT-R-Ig or hp80 TNFR-Ig. The conditions for all experiments were: 25°C, flow rate of 10 μl / ml min.
[0127] All experiments used HBS buffer (10 mM HEPES, 150 mM NaCl, 0.005% P20 surfactant, pH 7.4). This solution is also used as running buffer and sample diluent. .
[0128] First, the surface of a CM5 chip (BIAcore, Inc.) was activated with N-hydroxysuccinimide / N-ethyl-N'-(3-diethylaminopropyl)-carbodiimide hydrochloride (BIAcore). Dilute hBCMA-Ig, hLT-R05-Ig, hp80-TNFR with 10mM acetic acid to 30g / ml, take 20μl, 15μl and 10μl of these three solutions respectively, then use 30μl and then 15μl ethanolamine-HCl (pH8.5) closed. This results in a surface density of 1600-3700 resonance units (RU). Chips were regenerated with 20 μl 1 mM formic acid. These were repeated 5 times to establish a repeatable stable baseline.
[0129] In the experiment, 100 μl each of myc-mApril, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com