Composition comprising tumor cells and extracts and method for using thereof
A technology of tumor cells and compositions, applied in the field of compositions containing tumor cells and extracts and its use, can solve the problems of reduced surface expression, inability to induce T helper cells, and restriction of T cell recognition
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108]Low-dose cyclophosphamide (CY) was administered to 64 patients with metastatic melanoma, and then these patients were treated with the melanoma vaccine prepared according to the above method, and the immunotherapy efficacy and antitumor activity were monitored. On day 0, the patient was administered intravenous cyclophosphamide 300Mg / M 2 . Three days later, they were given an intradermal injection of a vaccine consisting of 10 x 10 6 to 25×10 6 Consisting of autologous, cryopreserved, irradiated (2500R) tumor cells; obtained by enzymatic (collagenase and DNase) dissociation of metastatic tumor masses. The sequence of treatments was repeated every 28 days for a total of eight treatments.
[0109] Following administration of cyclophosphamide, the toxicity of the treatment was limited to a local inflammatory response at the injection site and mild nausea and vomiting. There were 40 evaluable patients with measurable metastases; 5 of them responded, 4 of which were compl...
Embodiment 2
[0114] Metastatic melanoma patients were sensitized to DNP by topical application of 1% dinitrochlorobenzene (DNCB) or dinitrofluorobenzene (DNFB) to the upper arm. After 2 weeks, they were given a vaccine consisting of 10 x 10 mixed with BCG 6 to 25×10 6 Consisting of autologous, irradiated melanoma cells conjugated to DNP; 3 days prior to administration of DNCB (or DNFB) or vaccine, the patient was given intravenous cyclophosphamide 300Mg / M 2 . After 2 vaccine treatments (8 weeks), a strong inflammatory response developed in the tumor mass of 3 out of 4 evaluable patients. Patient #1 developed erythema and swelling in >50 large (1-3 cm) skin metastases on her legs and lower abdomen, followed by ulceration and drainage of necrotic material, some of which began to resolve. Biopsy showed that CD4+CD8+ T lymphocytes had infiltrated the tumor. Patient 2# developed erythema and swelling in the skin covering a large (8 cm) nodular mass in her lower abdomen and groin. These tum...
Embodiment 3
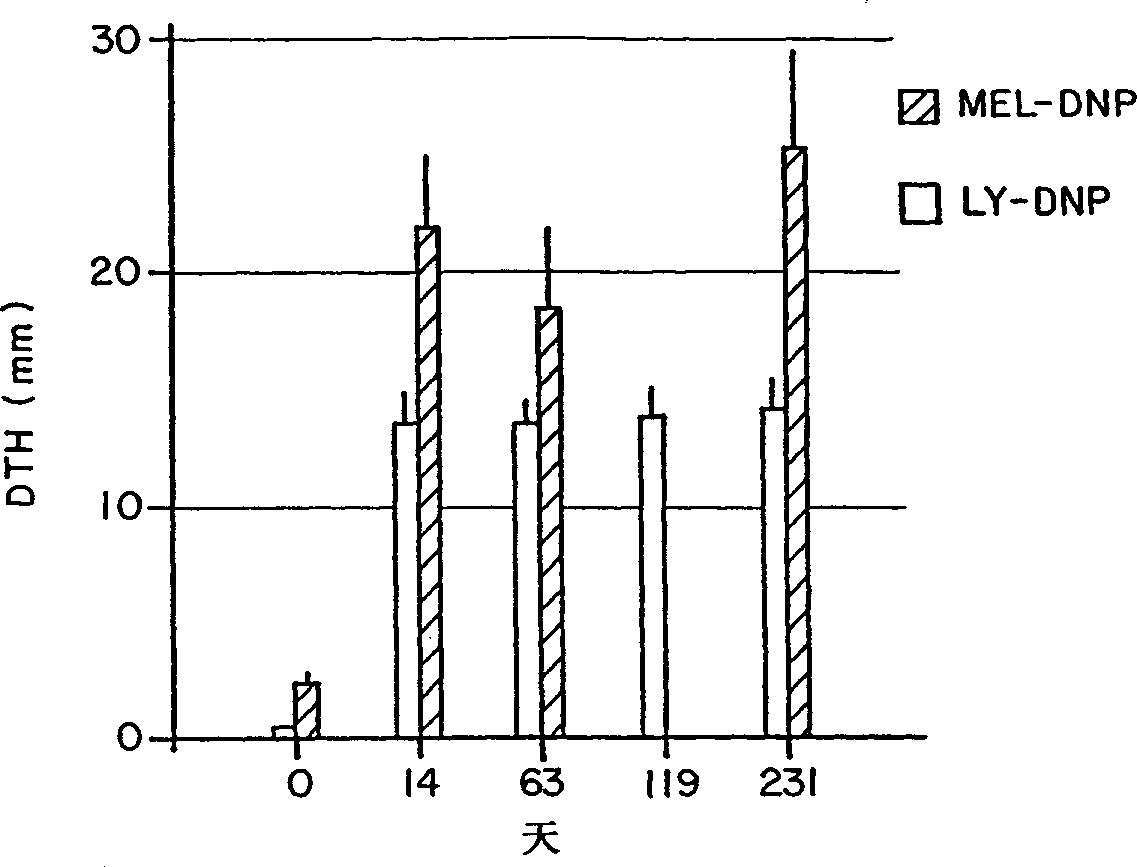
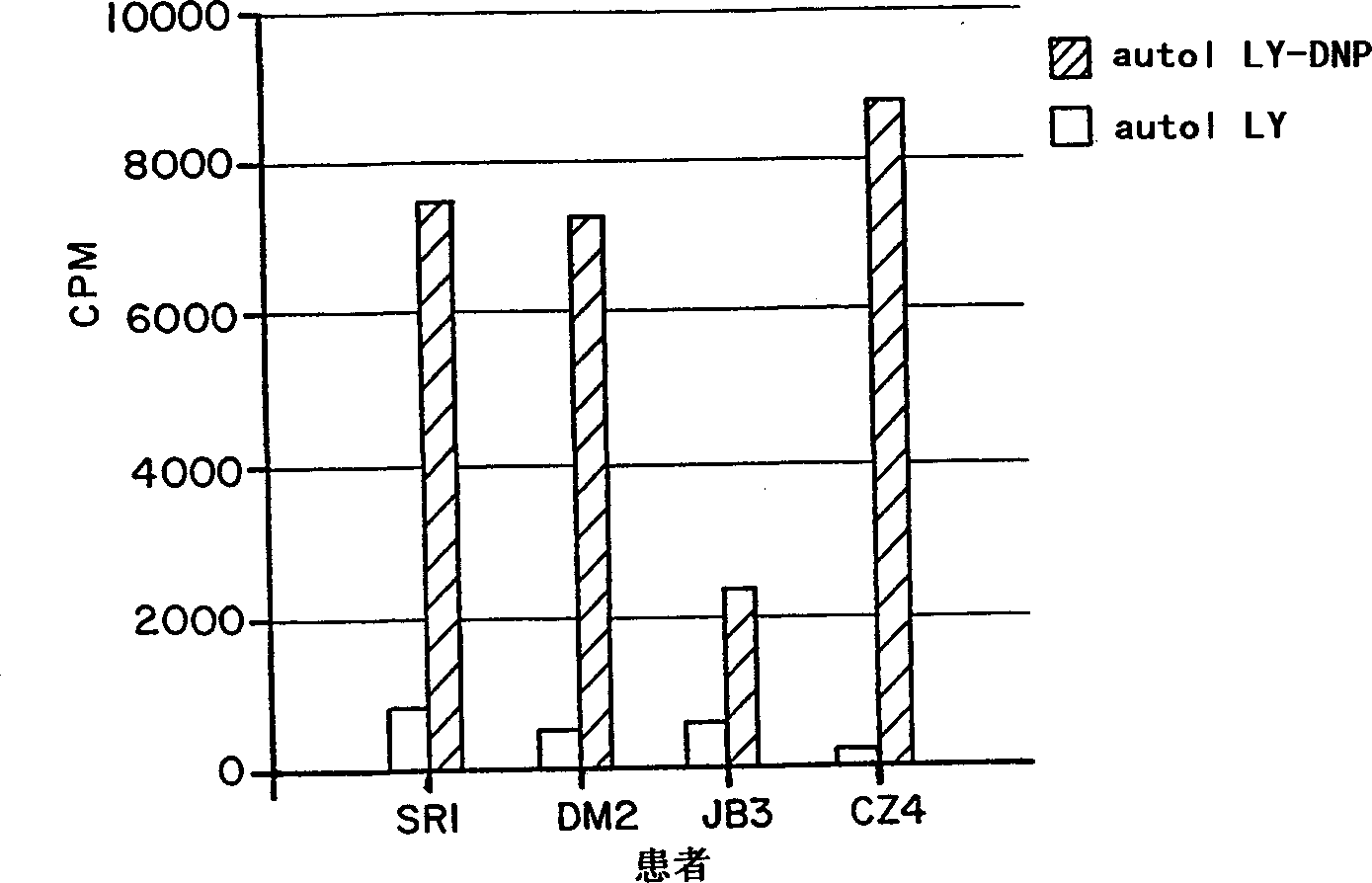
[0115] Fifteen patients (including 3 patients in Example 2) were treated with metastatic melanoma using a new form of immunotherapy, a tumor cell vaccine conjugated to DNP. Sensitization of metastatic melanoma patients to DNP by topical application of 5-dinitrochlorobenzene in the upper arm. Then give the patient cyclophosphamide 300Mg / M every 4 weeks 2 , 3 days later, the patient was injected with DNP-conjugated 10 × 10 6 to 25×10 6 Autologous, irradiated melanoma cells. Patients received 6-8 treatments. Most patients (92%) developed delayed-type hypersensitivity reactions (mean DTH = 17 mm) to autologous lymphocytes or tumor cells conjugated to DNP. In 11 / 15 patients, the vaccine induced a surprising inflammatory response in subcutaneous and nodal metastases consisting of erythema, swelling, warming and softening around the tumor mass and in one case pus Sexual drainage. Biopsy showed lymphocytic infiltration, which was mainly CD3+, CD4-, CD8+, HLA-DR+ T cells by immun...
PUM
 Login to view more
Login to view more Abstract
Description
Claims
Application Information
 Login to view more
Login to view more - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap