Microencapsulation method of Chinese traditional medicine
A technology of traditional Chinese medicine and capsule materials, applied in the directions of microcapsules and capsule transportation, can solve the problems of unstable drug content, large resistance of drug preparations, and harsh production environment, so as to improve the fluidity and compressibility of drugs, and improve bioavailability. degree, overcome the effect of sublimation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Take 10g of borneol, dissolve it in 100ml of ethanol, and add 3g of polyvinylpyrrolidone. Microcrystalline cellulose 1g, ultrasonically mixed, added dropwise to 500ml aqueous solution containing 0.5g each of gelatin and gum arabic. When adding dropwise, stir the water phase to make it fully mixed, ultrasonically disperse for 30 minutes, adjust the pH value to 4, react for 1 hour, add a cross-linking agent, that is, a total of 5ml of 20% formaldehyde solution and 5% glutaraldehyde solution, Adjust the pH value to 8, cross-link and solidify for 2 hours to obtain borneol microcapsules. The filtered product was washed with water, and post-treated with 20 ml of 5% citric acid solution for 10 hours to obtain borneol microcapsules with an average particle size of 50-60 um.
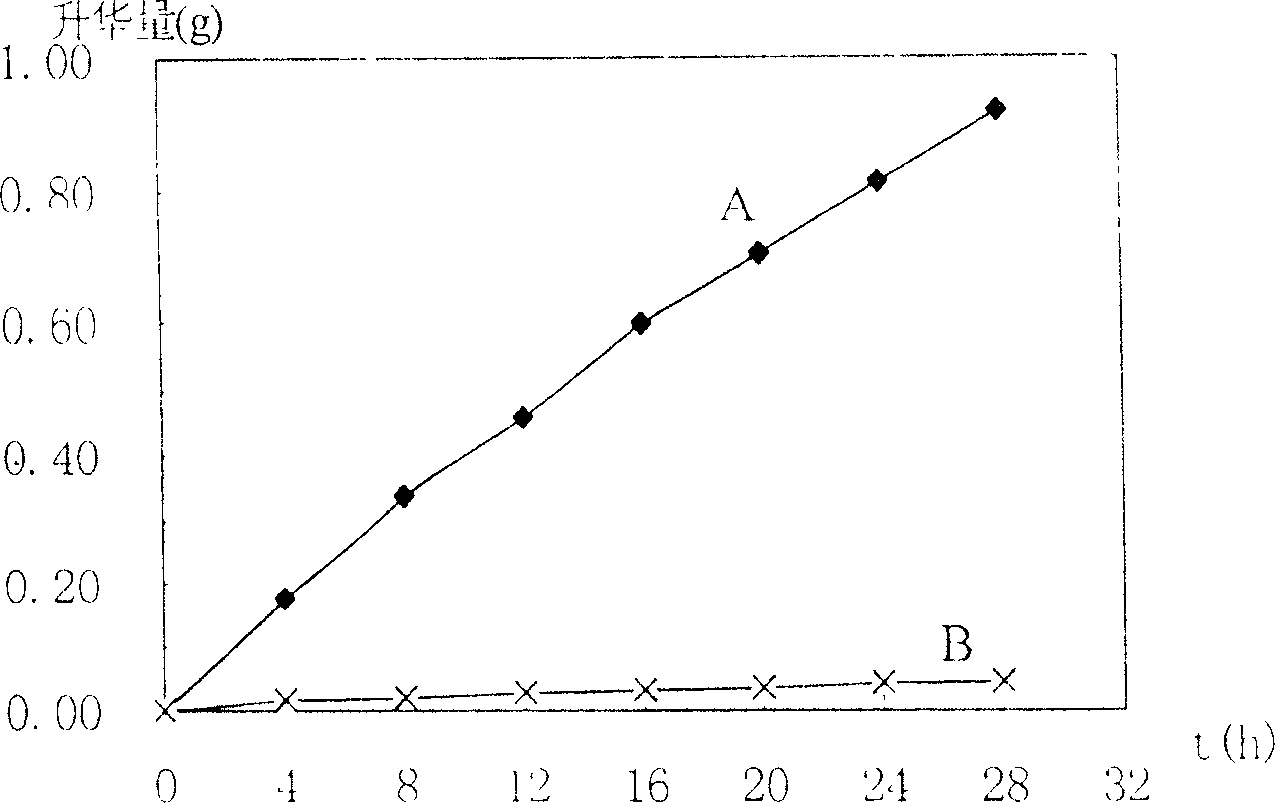
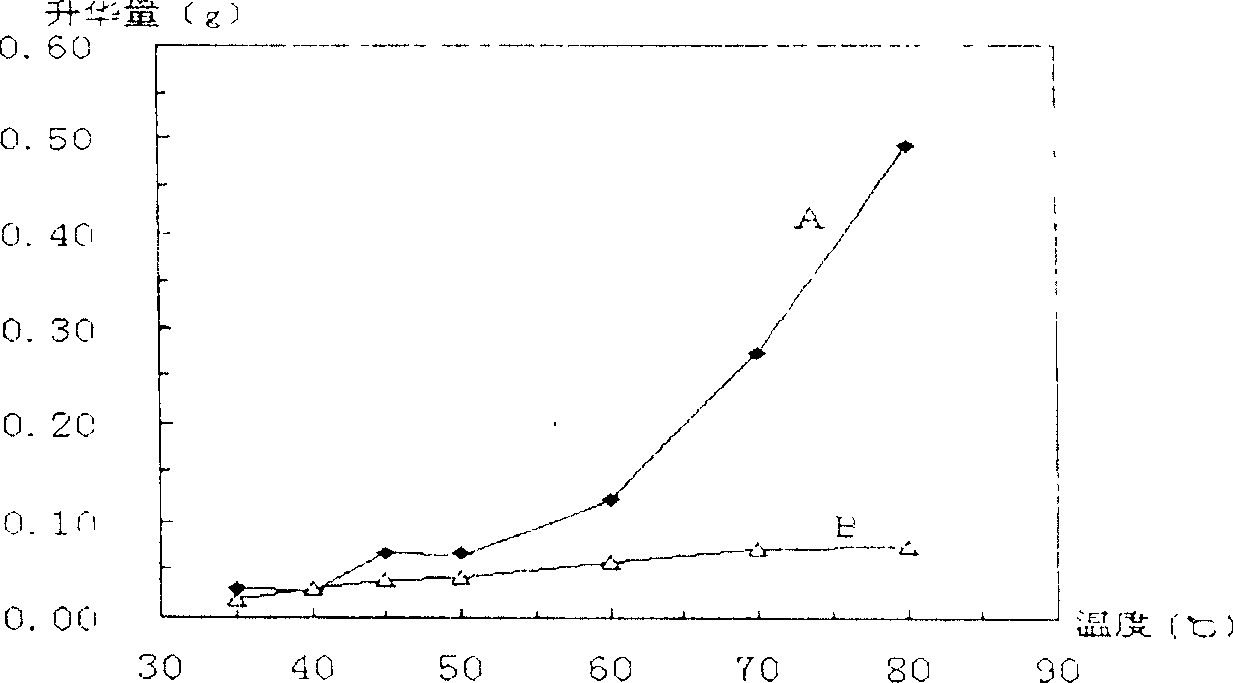
[0023] The efficacy of encapsulating drug-loaded microcapsules is as follows: figure 1 and figure 2 shown. figure 1 It is a comparison chart of the sublimation amount and time relation curve of borneo...
Embodiment 2-4
[0025] Take 10 g of sage oil (or cane oil, or cocklebur seed oil) and 1 g of ethyl cellulose, dissolve them in 50 ml of ethyl acetate, and prepare an oil phase. 1 g of sodium lauryl sulfate was dissolved in 100 ml of water, and 1 ml of ethyl acetate was added to prepare an aqueous solution saturated with ethyl acetate, which was used as the water phase. Under the condition of stirring the water phase, drop the oil phase into the water phase at a constant speed, cross-link and solidify for 2 hours, then add 500ml of water, remove the solvent, and then the oily oil (or Atractylodes atractylodes oil, or Cocklebur seed oil) can be formed. Microcapsules. Then filter and dry to obtain microcapsules. The average particle size of microcapsules is 50-60um. The encapsulation rate is 99%.
Embodiment 5
[0027] Take 10 g of Chuanxiong oil and 1 g of polyacrylic acid resin, and dissolve them in 50 ml of ethyl acetate to prepare an oil phase. Take 100ml of water, add 2g of polyethylene glycol, and sonicate for 30 minutes to prepare an aqueous phase. Under the condition of stirring the water phase, drop the organic phase into the water phase at a uniform speed, solidify for 3 hours, remove the solvent, filter and dry to obtain the drug-loaded microcapsules of Rhizoma Chuanxiong. Others are with embodiment 2-4. The average particle size is 50-60um. The encapsulation rate is 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com