Pharmaceutical compositions contg. anti-body-enzyme conjugates in combination with prodrugs
A technology of prodrugs and conjugates, applied in drug combinations, medical preparations containing active ingredients, nano-drugs, etc., can solve optimization difficulties, difficulties in enzyme expression duration and reproducibility, and lack of specificity in enzyme expression And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
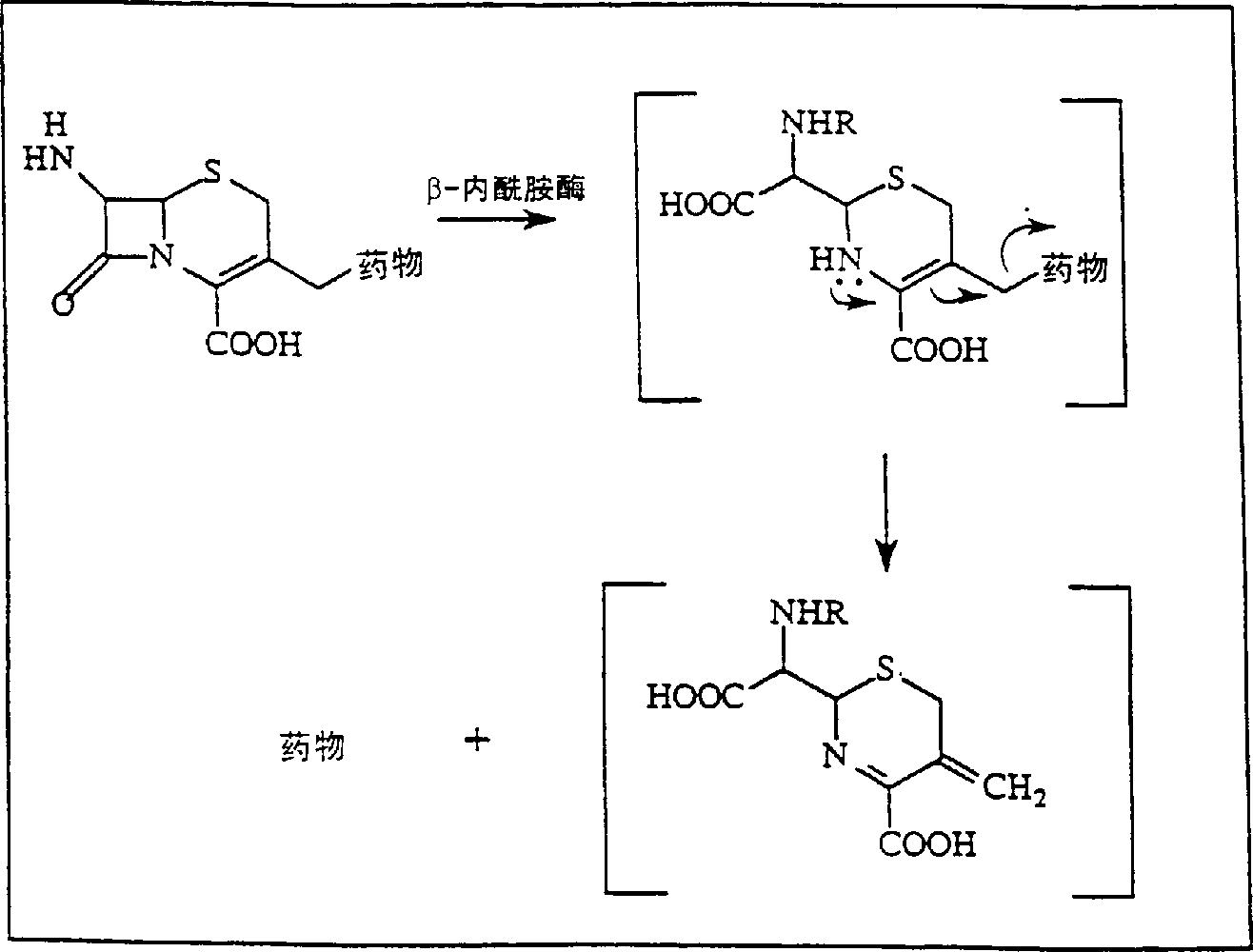
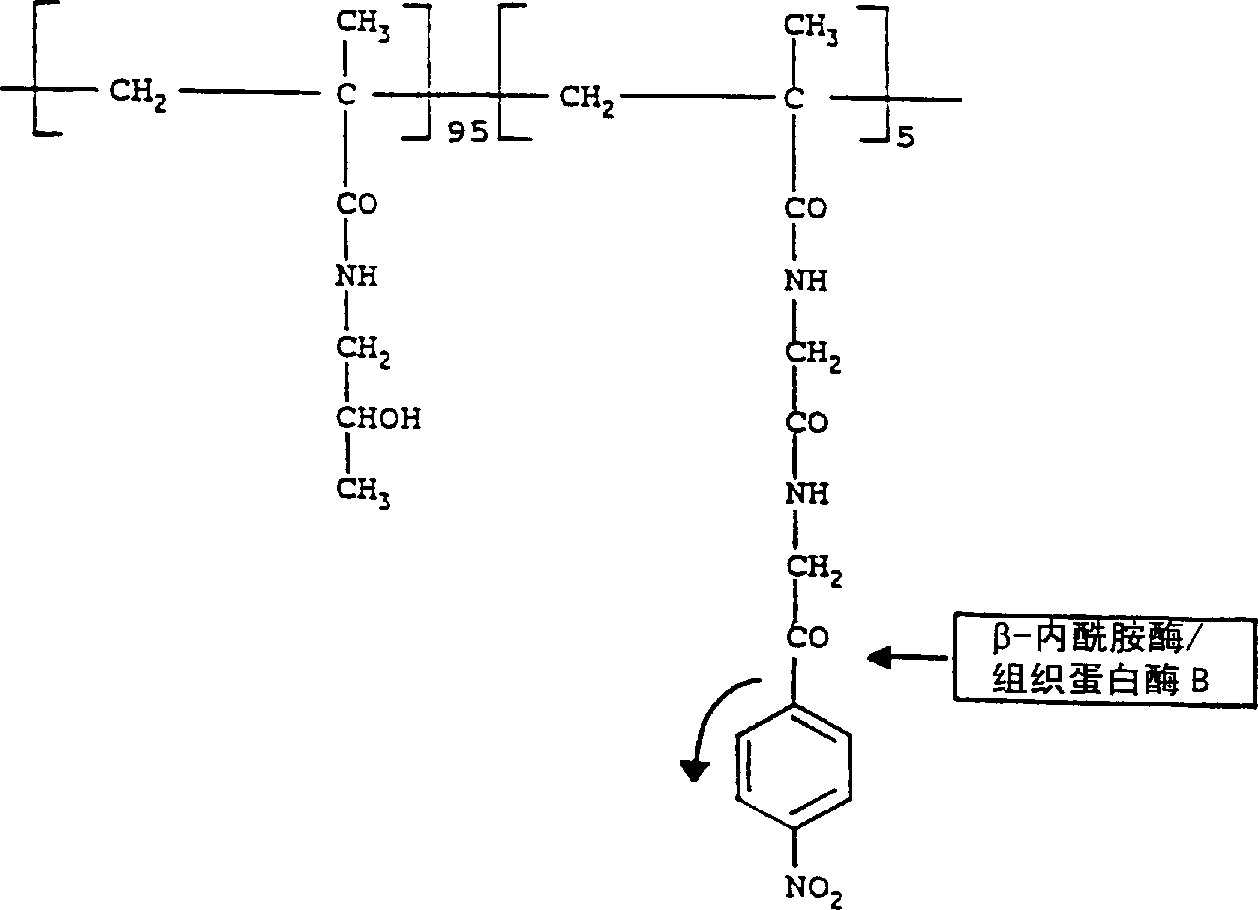
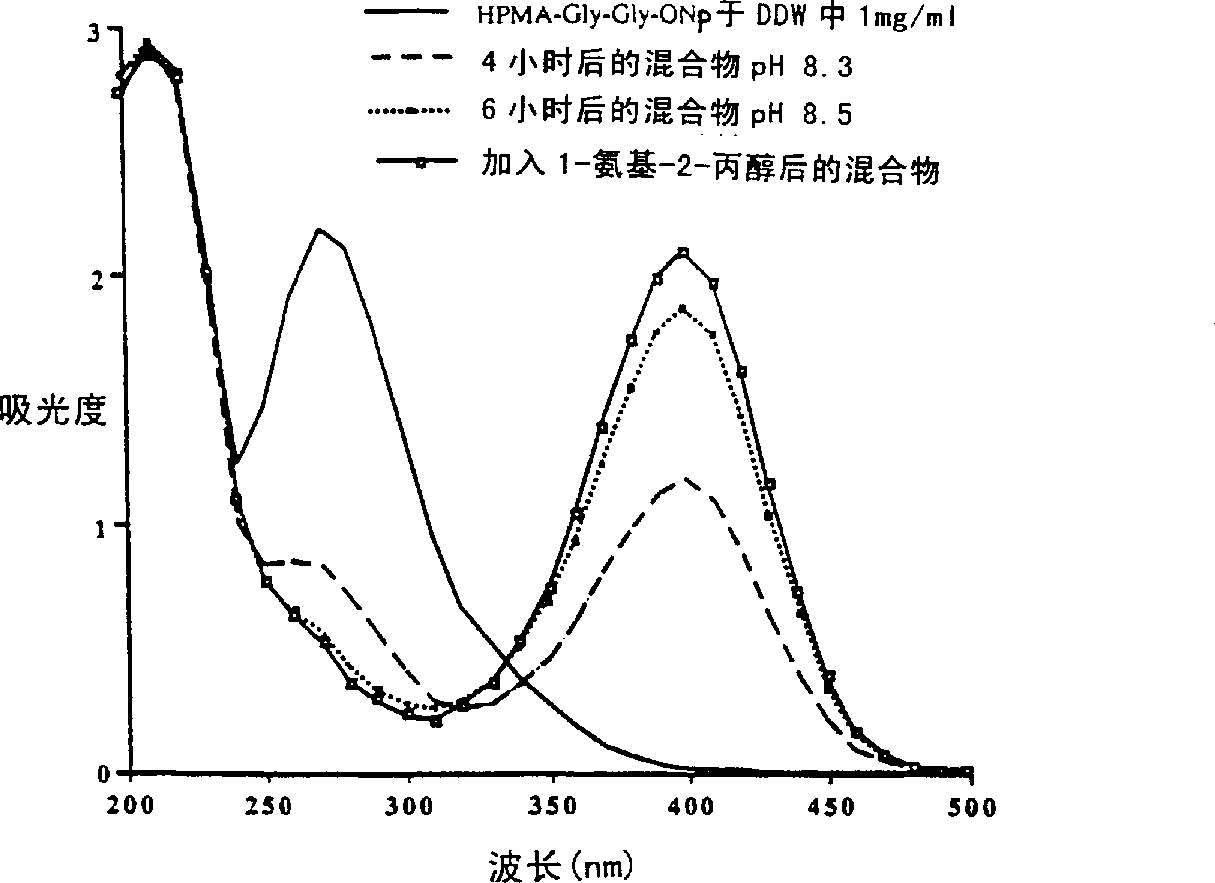
[0081] Invention with reference to the accompanying drawings figure 2 etc. are further illustrated in the following examples. Example 1: HPMA-β-lactamase conjugates
[0082] β-lactamase (Sigma; ~29kDa) reacts with the polymer carrier N-(2-hydroxypropyl)methacrylamide-glycine-glycine-P - Nitrophenol (HPMA-Gly-Gly-ONp; Polymer Laboratories; ~30 kDa) bound. react in figure 2 Indicated. The following steps were performed:
[0083] Dissolve the polymer with side chain P-nitrophenol (ONp) group in double distilled water (4mg / ml) and dissolve the β-lactamase in 0.05M phosphate buffer at pH 7.2 (2mg / ml), and this solution was added to the polymer solution at 4°C with stirring.
[0084] The reaction mixture was stirred at pH 7.2 for 30 minutes in the dark. The pH was carefully raised to 8.5 over 4 hours (to prevent enzyme denaturation) by the addition of saturated sodium tetraborate buffer, and the reaction mixture was stirred for an additional 4 hours. The reaction was termi...
Embodiment 2
[0101] Cathepsin B (Sigma, ~28 kDa) was bound to the polymeric carrier (HPMA-Gly-Gly-ONp; ~30 kDa) by nonspecific aminolysis of the ONp group at controlled pH. react in figure 2 Indicated. The same procedure as in Example was carried out except that the polymer was dissolved in double distilled water at 1 mg / ml and cathepsin B was used instead of β-lactamase.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com