Myocardial cell resuscitation method
A technology of cardiomyocytes and human serum albumin, applied in the field of cardiomyocyte resuscitation, can solve the problems of cardiomyocyte myocardial contractility reducing electrophysiological activity, affecting the stability of cardiomyocytes, and no method of cryopreservation or resuscitation of cardiomyocytes has been found. Achieve stable electrophysiological activity and high activity rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Example 1 Preparation of cardiomyocyte cryopreservation solution
[0023] 1: Prepare a cardiomyocyte cryopreservation solution containing 90% fetal bovine serum and 10% DMSO;
[0024] 2: Dissolve 0.8 g of hydroxyethyl starch in 70 mL of physiological saline with a concentration of 0.9%, and then add 0.3 mL of DMSO and 30 mL of human serum albumin after it is completely dissolved;
[0025] 3: Dissolve 1 g of hydroxyethyl starch in 75 mL of physiological saline with a concentration of 0.9%, and then add 0.5 mL of DMSO and 25 mL of human serum albumin respectively after it is completely dissolved;
[0026] 4: Dissolve 5g of hydroxyethyl starch in 80mL of physiological saline with a concentration of 0.9%, and then add 10mL of DMSO and 10mL of human serum albumin respectively after it is completely dissolved;
[0027] 5: Dissolve 1 g of hydroxyethyl starch, 0.1 μmol of ROCK inhibitor Y27632, and 0.25 g of glucose in 70 mL of physiological saline with a concentration of 0.9%...
Embodiment 3
[0042] Example 3 The effect of cryopreservation on myocardial cell viability
[0043] The myocardial cell viability was calculated by PI staining method for the cryopreserved and resuscitated cardiomyocytes. Table 1 shows the effects of different cardiomyocyte cryopreservation solutions for 6 months on the viability of cardiomyocytes differentiated to the 8th day:
[0044] Table 1: Cell viability of cardiomyocytes on day 21 of differentiation in different cardiomyocyte cryopreservations for 6 months
[0045] cryopreservation 1 2 3 4 5 6 7 8 cell viability 47.25% 65.5% 72.5% 72.75% 83.25% 81.75% 91.5% 93.25%
[0046] It can be seen that, compared with the cardiomyocyte cryopreservation solution of group 1 in Example 1 of the present invention, groups 2 to 8 provided in the embodiment of the present invention significantly improved the viability of cardiomyocytes. Cardiomyocytes were frozen in the cardiomyocyte cryopreservation solution of group...
Embodiment 4
[0048] Example 4 The effect of cryopreservation on the electrophysiological activity of cardiomyocytes
[0049] Patch-clamp detection and recording of electrophysiological properties of beating cardiomyocytes
[0050] The same batch of cardiomyocytes with the same maturity were stored in the cryopreservation solution provided in Example 1 for 12 months, then recovered and cultured in cardiomyocyte culture medium, and pulsating myocardium was selected after culturing for 48h. The cells were also subjected to patch-clamp experiments, in which the cardiomyocyte culture medium was a purchased commercial cardiomyocyte culture medium.
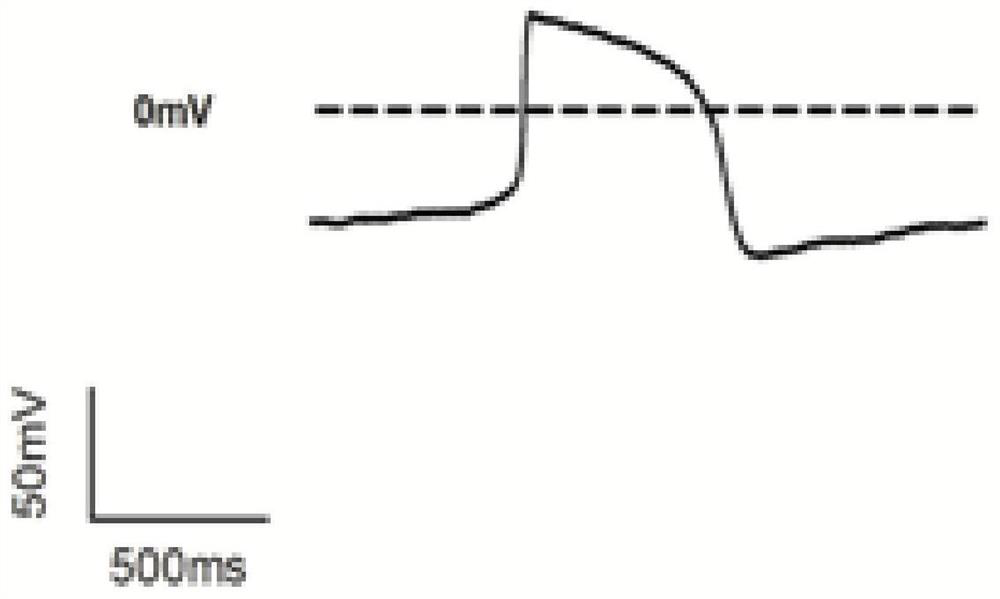
[0051] figure 1 It is the action potential of unfrozen cardiomyocytes, showing that its action potential duration APD is 416.10 ± 32.61, and the action potential duration APD of the cardiomyocytes frozen in the first group of cryopreserved solution in Example 1 is 265 ± 36.98. In Example 1, the action potential durations of the cardiomyocytes cryop...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com