Anti-human Cripto-1 antibody
A technology of antibodies and monoclonal antibodies, applied in the direction of antibodies, anti-animal/human immunoglobulins, anti-tumor drugs, etc., can solve problems that are unlikely to cure cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
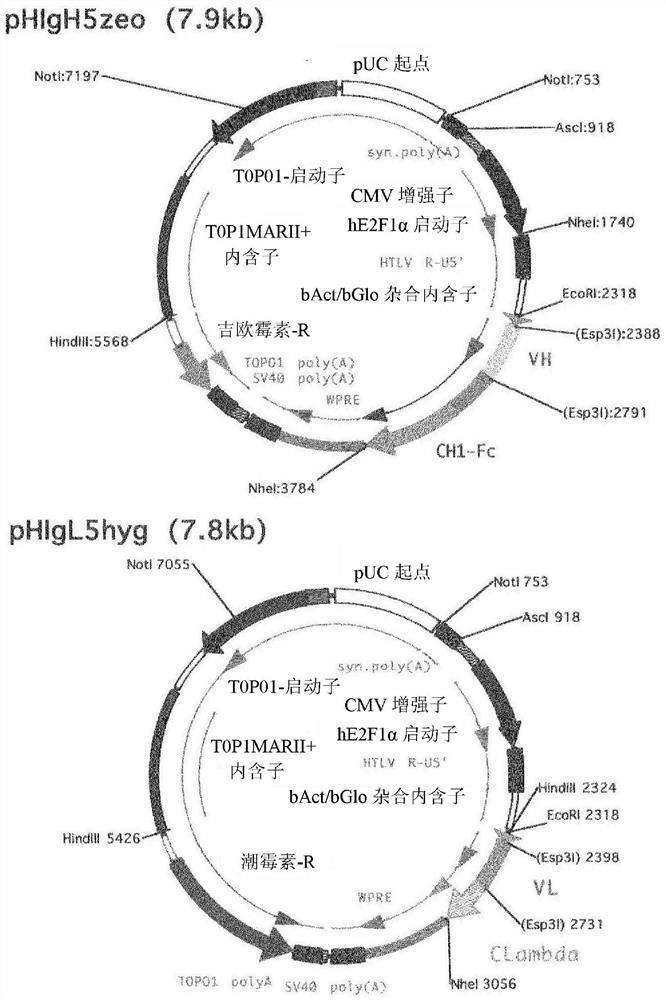
[0180] Example 1: Screening of anti-human Cripto-1 antibodies
[0181] Phage-displayed human antibody libraries were screened for antibodies that specifically recognized the extracellular domain of human Cripto-1. The extracellular domain of human Cripto-1 contains the amino acid sequence represented by SEQ ID NO: 11, and has a His-tag sequence having the amino acid sequence represented by SEQ ID NO: 12 at its N-terminus. The extracellular domain of human Cripto-1 was prepared in its soluble form according to the method described in Alam. et.al., Int J Mol Sci. 2018, 26; 19(11). Antibody libraries used as screening targets are described in Sakai, K. et al., Biochemistry 46(1):253-62, and the subclass of antibodies is IgG.
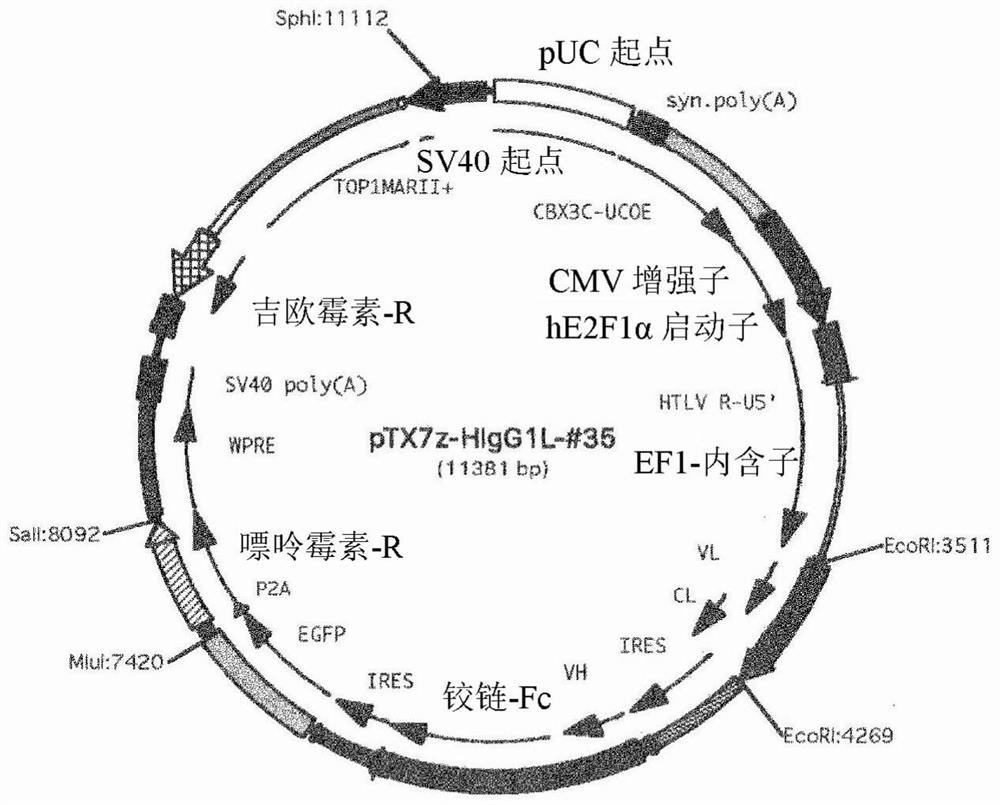
[0182] As a result, the #35 cloned phage strain was obtained as an antibody that specifically recognized the extracellular domain of purified human Cripto-1. The nucleotide sequence encoding the antibody was analyzed based on the clone, and based on the...
Embodiment 2
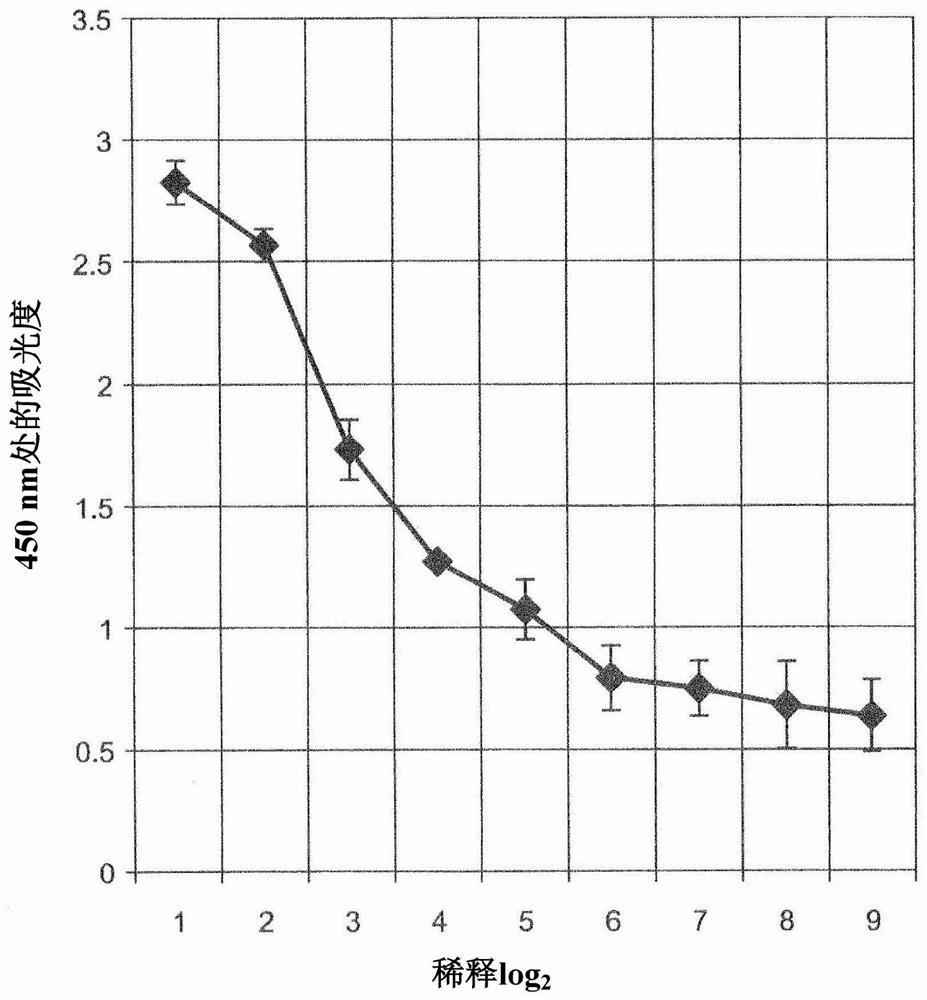
[0186] Example 2: Evaluation of antigen recognition ability by ELISA
[0187] The culture supernatant of clone #35 antibody was collected and diluted 25-fold with phosphate buffer, followed by further serial dilutions every 2-fold. The diluted culture supernatant was added to a 96-well plate, and IgG molecules (anti-human Cripto-1 antibody of human origin) adsorbed on each well were detected with HRP-labeled anti-human IgG goat antibody (manufactured by Abcam) . figure 2 Results are shown.
[0188] like figure 1 As shown in , the staining by HRP decreased with the degree of dilution of the culture supernatant containing the #35 clone antibody. This indicates that the #35 clone antibody is specifically adsorbed to its antigen, human Cripto-1.
Embodiment 3
[0189] Example 3: Evaluation of specificity against (human) cancer tissue using tissue arrays
[0190] Immunostaining was performed on human cancer tissue array (BCN801, US Biomax Inc.). Clone #35 was used as the primary antibody. ALEXA568-labeled anti-human IgG goat antibody (Invitrogen) was used as secondary antibody to detect each cancer tissue (pancreatic cancer tissue, thyroid cancer tissue, prostate cancer tissue, gastric cancer tissue, cervical cancer tissue, colorectal cancer tissue and breast cancer tissue tissue) in human Cripto-1. These cancer tissues contain cancer stem cells as well as cancer cells, and it is believed that Cripto-1 is bound to the surface of the cell membrane of cancer stem cells through a GPI-anchor. In negative control experiments, signal background was determined by using only the secondary antibody. Figures 3 to 5 Results are shown.
[0191] like Figures 3 to 5 Shown in , staining images of the #35 clone antibody were identified in al...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com