Compositions and methods for treating alzheimer's disease
A technology of Alzheimer's disease and composition, which is applied in the field of composition for treating Alzheimer's disease, and can solve problems such as effective treatment of Alzheimer's disease and unmet needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0088] Example 1: Preparation of Nanoemulsion
[0089] Nanoemulsions were prepared according to the formulations in the table below and the following method.
[0090]
[0091] Oil phase: medium chain triglyceride oil (triglycercide oil) and lecithin were mixed in a 10 mL beaker at 300 rpm for 2 hours at 60°C. Melatonin and THC were added after the lecithin was completely dissolved and stirred for another hour at 300 rpm and 60°C.
[0092] Insulin: Suspend 15.58mg (28.8U / mg) insulin in 1mL H 2 O and 5 μL of 12N HCl, and water was added until the insulin was completely dissolved (total volume approximately 1.3 mL).
[0093] Aqueous Phase: The following compositions were prepared in a 40 mL beaker and mixed for 2 hours at 300 rpm and room temperature.
[0094] 1% Methylparaben = 0.15mg
[0095] 1% Propylparaben = 0.15mg
[0096] 1% Pluronic F = 0.15mg
[0097] 2.5% Glycerol = 0.375mL
[0098] 5% PEG 400=0.75mL
[0099] water until the total volume is about 9.19mL
...
Embodiment 2
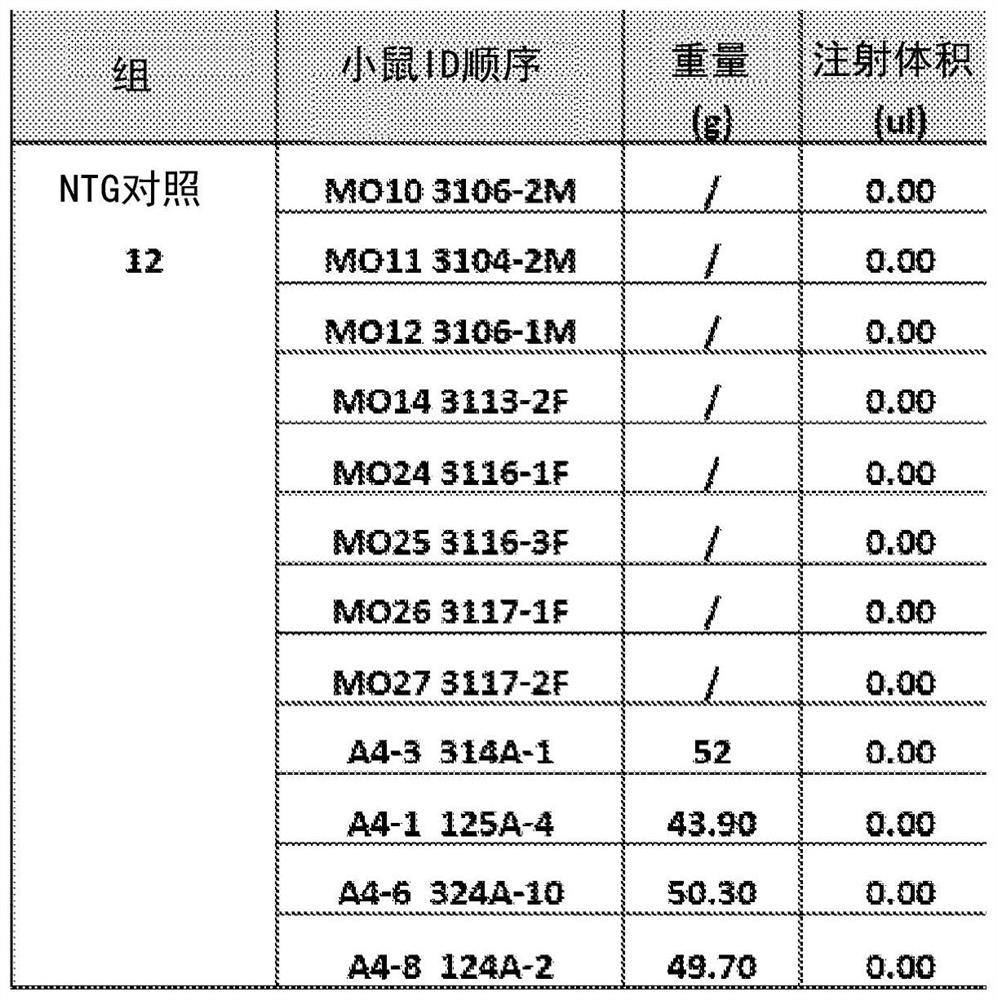
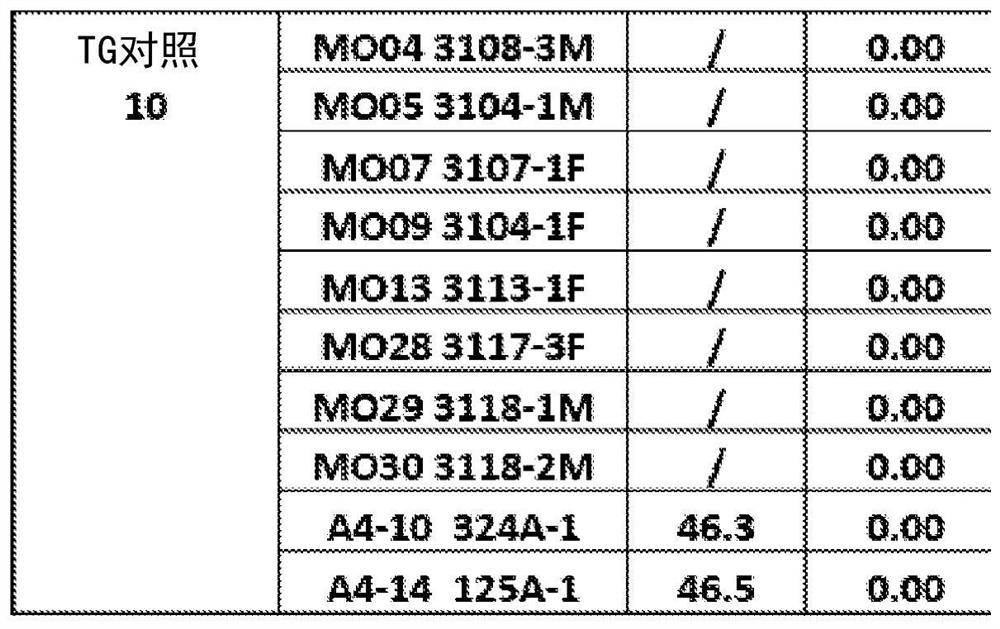
[0109] Example 2: Tg mice were impaired in memory tests prior to treatment
[0110] The memory of the mice was tested using RAWM, and the results showed that the TG mice had memory impairment. Mice were grouped according to pre-treatment behavioral outcomes and blood amyloid beta levels. Figures 2A-2B are the pre-treatment behavioral results in NTG and TG mice. Figure 2A is a graph depicting a mouse making mistakes in a RAWM pool, Figure 2B is a graph depicting the delayed results of mice finding a platform in a RAWM pool. T1 and T5 denote Trial 1 and Trial 5, respectively. Compared to Trial 1, NTG mice in Trial 5 improved significantly, but there was no difference in TG mice. therefore, Figures 2A-2B showed that TG mice had memory impairment. Figures 3A-3B Grouping results based on plasma Aβ levels and pre-treatment behavioral outcomes are described. Figure 3A No difference was shown between the TG Ctr group and the TG TMI group. Figure 3B are pre-behavioral gr...
Embodiment 3
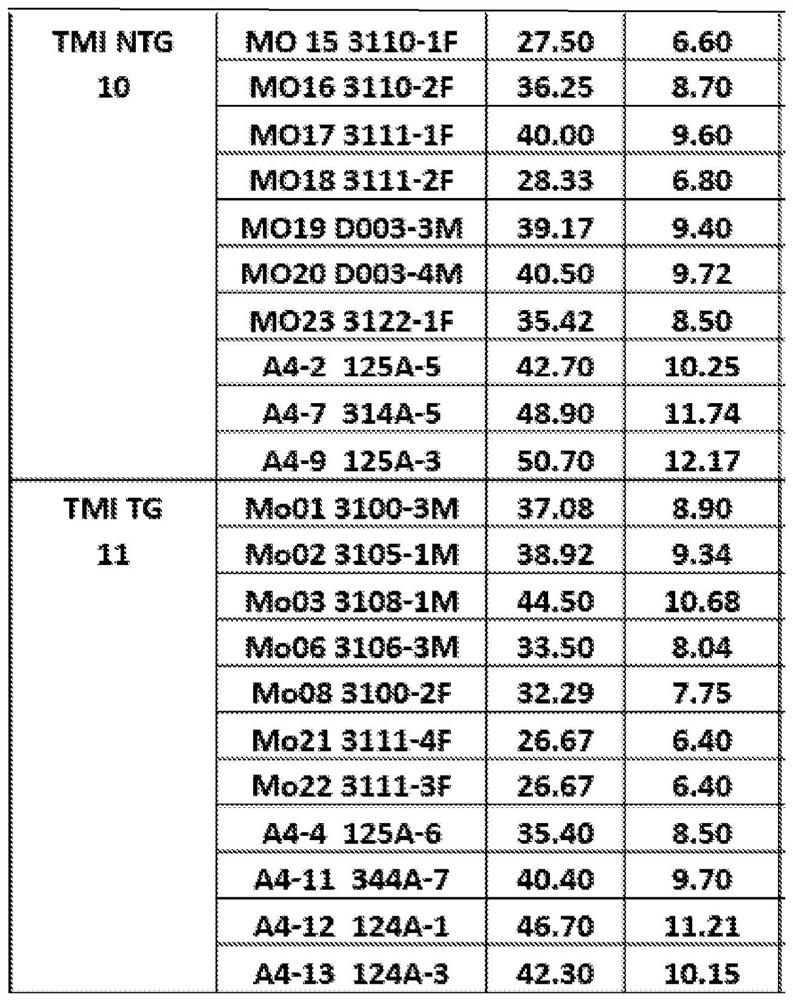
[0111] Example 3: TMI treatment can slow memory decline in TG mice
[0112] Memory tests were performed after 3 months of TMI treatment, demonstrating that TMI reduced errors in both TG and NTG mice. Figures 4A-4B Behavioral test results by RAWM after treatment are shown. Figure 4A A graph showing the miss test results for T1 and T5. exist Figure 4A In the NTG Ctr (first panel), NTG TMI (second panel) and TG TMI (fourth panel) groups, there was a significant difference between Trial 1 and Trial 5, but not in TG Ctr (third panel). Figures) did not differ among groups. Figure 4B is a series of graphs depicting differences in latency across multiple groups. exist Figure 4B , the first graph is NTG Ctr, the second graph is NTG TMI, the third graph is TG Ctr, and the fourth graph is TG TMI. Figure 4B It was shown that when comparing Trial 1 and Trial 5, there was no change in latency for all groups. (NTG Ctrn=9, NTG TMI n=9, TG Ctr n=10, TG TMI n=9).
[0113] These ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com