In-vitro release detection method of ripdicaine emulsifiable paste
A technology of risprocaine and detection method, which is applied in the field of in vitro release detection of risprocaine cream, can solve the problems of affecting the discrimination of release methods and low repeatability of in vitro release, so as to reduce the difficulty of quality control, The effect of precise monitoring
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Using SOTAX CE7 flow cell, Merck Express PLUS PES filter membrane.
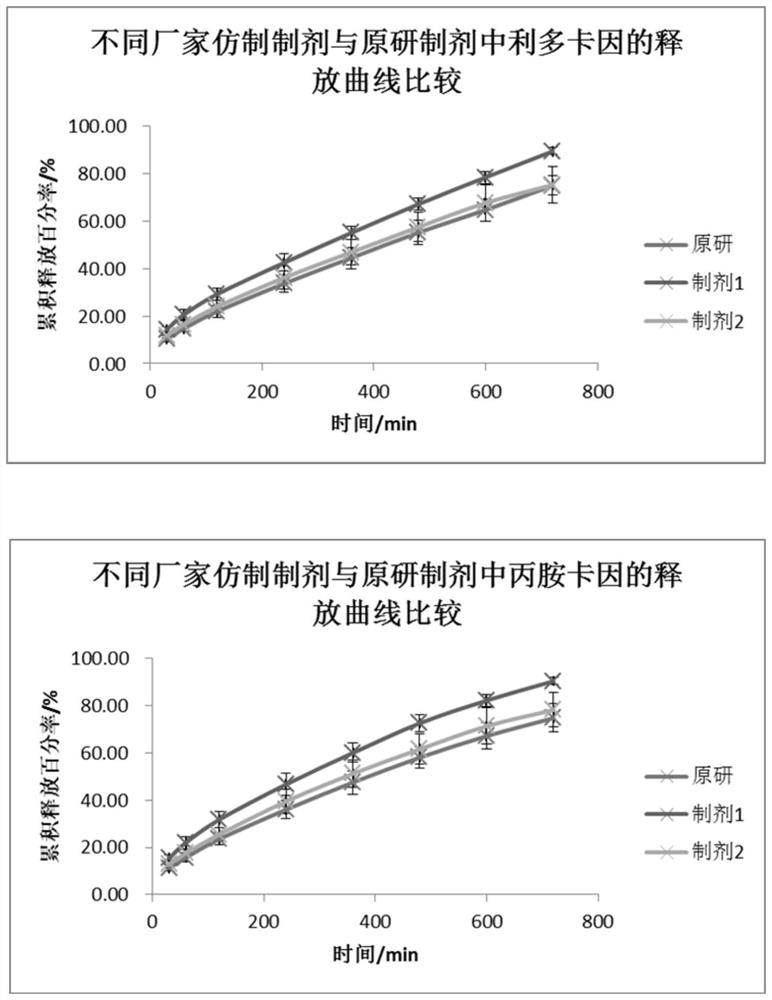
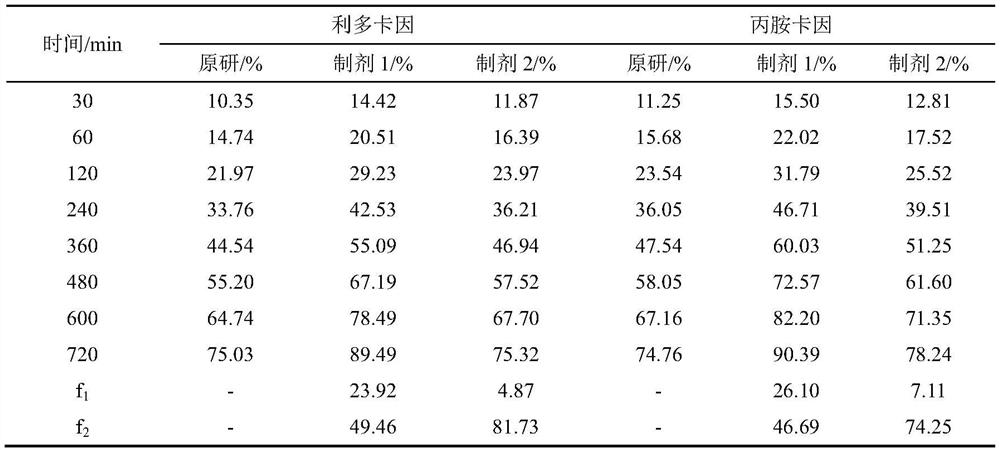
[0039]A flow cell method for measuring the in vitro release of lipradicaine emulsifiable paste comprises the following steps. The in vitro release of the original preparation and the generic preparations from two manufacturers were measured respectively.
[0040] (1) The SOTAX CE7 flow cell is used as the experimental device, and the system is set as a closed-loop system. The experimental temperature is 32°C, the flow rate is 16mL / min, and the sampling time is 15 / 30 / 60 / 120 / 240 / 360 / 480 / 600 / 720min.
[0041] (2) Pre-saturate the Merck Express PLUS PES filter membrane with freshly prepared PBS6.8 buffer solution for 30 minutes, add the sample into a 0.4mL cream pool (sample weight 350-400mg), scrape off the excess sample on the surface with a scraper, and put The pre-saturated membrane is taken out and the surface moisture is removed with absorbent paper, the filter membrane is put into the fixing ring,...
Embodiment 2
[0049] Using SOTAX CE7 flow cell, Merck Express PLUS PES filter membrane.
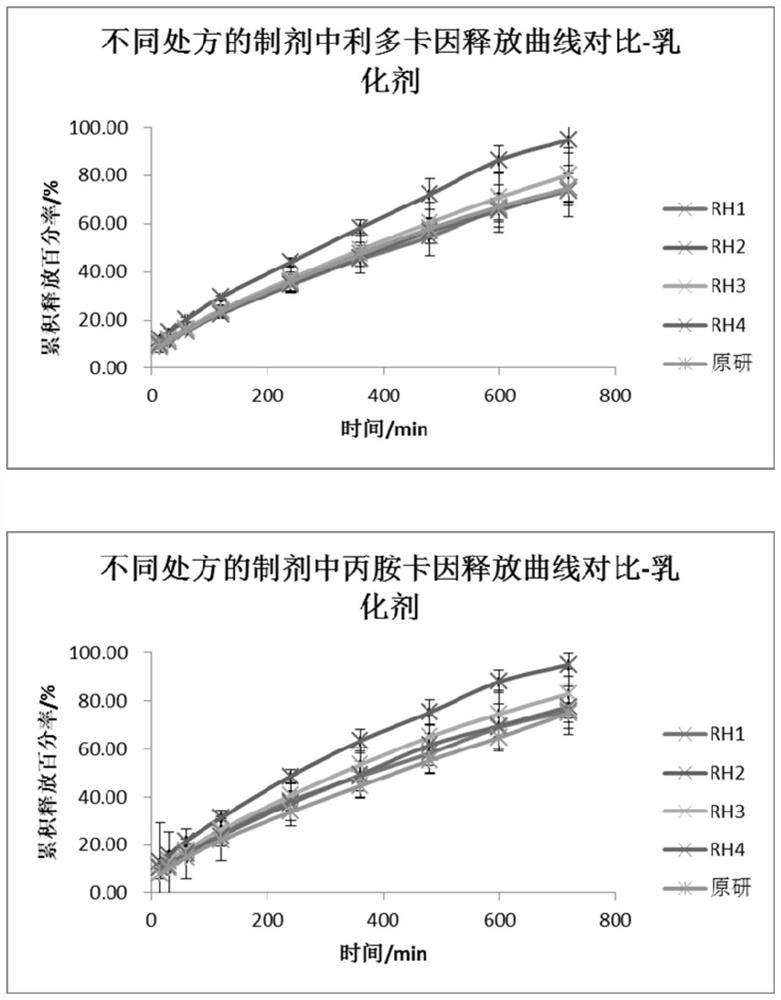
[0050] A flow cell method for measuring the in vitro release of lipradicaine emulsifiable paste comprises the following steps. This method was used to investigate the in vitro release behavior of the trial preparation obtained by changing the emulsifier in the formulation.
[0051] (1) The SOTAX CE7 flow cell is used as the test device, the system is set as a closed-loop system, the test temperature is 32°C, the flow rate is 16mL / min, and the sampling time is 15 / 30 / 60 / 120 / 240 / 360 / 480 / 600 / 720min.
[0052] (2) Pre-saturate the Merck Express PLUS PES filter membrane with newly prepared PBS6.8 buffer solution for 30 minutes, add the sample into a 0.4mL cream pool (the weighing sample size is 350-400 mg), and scrape off the excess on the surface with a scraper. For the sample, take out the pre-saturated membrane and remove the surface moisture with absorbent paper, put the filter membrane into the fixing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com