Oxazine near-infrared fluorescent dye as well as preparation method and application thereof
A fluorescent dye and near-infrared technology, which is applied in the direction of oxazine dyes, organic dyes, luminescent materials, etc., can solve the problem of high non-specific tissue uptake, and achieve the effect of improving specificity and improving specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
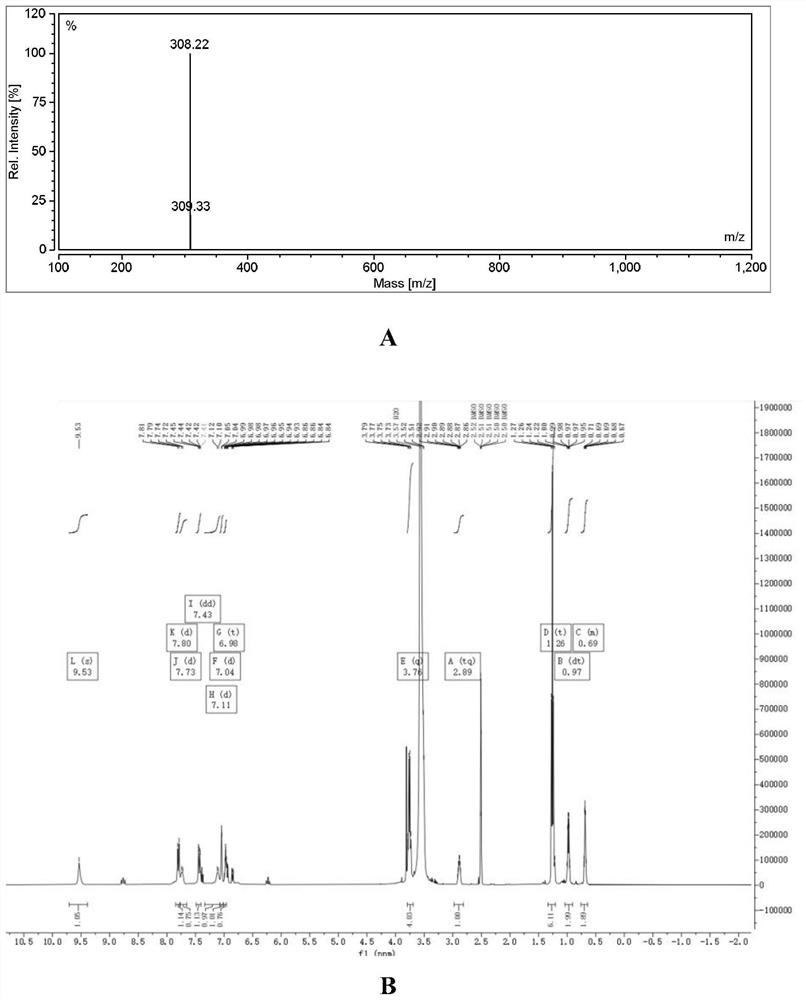
Embodiment 1
[0031] Synthesis of YQN-3 (i.e. compound I-1)
[0032] 1. Take 1.50g of m-bromoanisole, 0.73g of cyclopropylamine, 1.36g of potassium tert-butoxide, and 0.05g of tetrakis(triphenylphosphine)palladium in toluene (8ml) and heat to reflux at 80°C for 8h. After the reaction, the solution was spin-dried, and the remaining solid was purified by column chromatography to obtain N-cyclopropyl-3-methoxyaniline as a brown oily liquid. m / z: 164.14.
[0033] 2. Dissolve 200 mg of N-cyclopropyl-3-methoxyaniline in 2M ice-cold dilute hydrochloric acid solution (5 ml), add 103 mg of sodium nitrite into the solution while keeping the solution temperature below 5°C. After the addition, the solution was stirred for another 2 h, then it was dropped into a saturated potassium carbonate solution, extracted with ethyl acetate, the organic layer was dried with anhydrous sodium sulfate, and spin-dried to obtain a yellow-green solid N-cyclopropyl-3-methoxy Base-4-nitrosoaniline. m / z: 192.09.
[003...
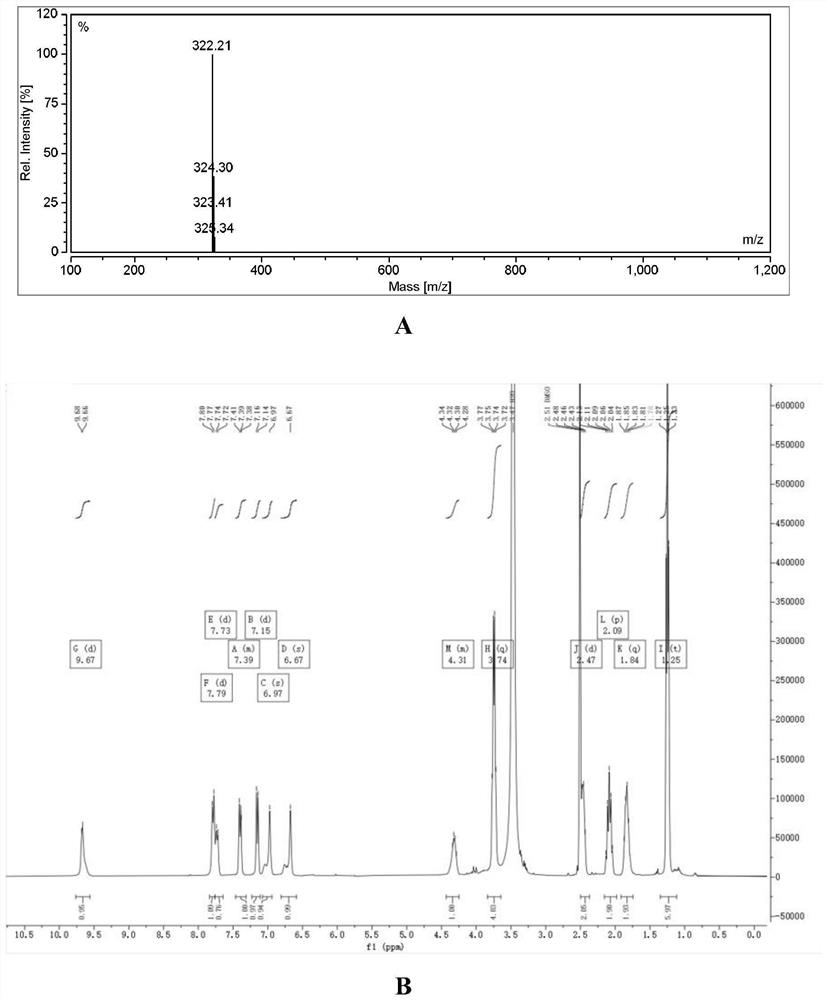
Embodiment 2
[0036] Synthesis of YQN-4 (i.e. compound I-2)
[0037] The synthesis of YQN-4 refers to Example 1. Its mass spectrometry and H NMR spectrum are as figure 2 As shown, the specific data are as follows: m / z: 322.21, 1H NMR (400MHz, DMSO-d6) δ9.67 (d, J=6.4Hz, 1H), 7.79 (d, J=9.4Hz, 1H), 7.73 ( d, J=9.3Hz, 1H), 7.46-7.31(m, 1H), 7.15(d, J=9.3Hz, 1H), 6.97(s, 1H), 6.67(s, 1H), 4.43-4.25(m , 1H), 3.74(q, J=7.1Hz, 4H), 2.47(d, J=9.3Hz, 2H), 2.09(p, J=9.6Hz, 2H), 1.84(q, J=8.6Hz, 2H ), 1.25(t, J=7.0Hz, 6H).
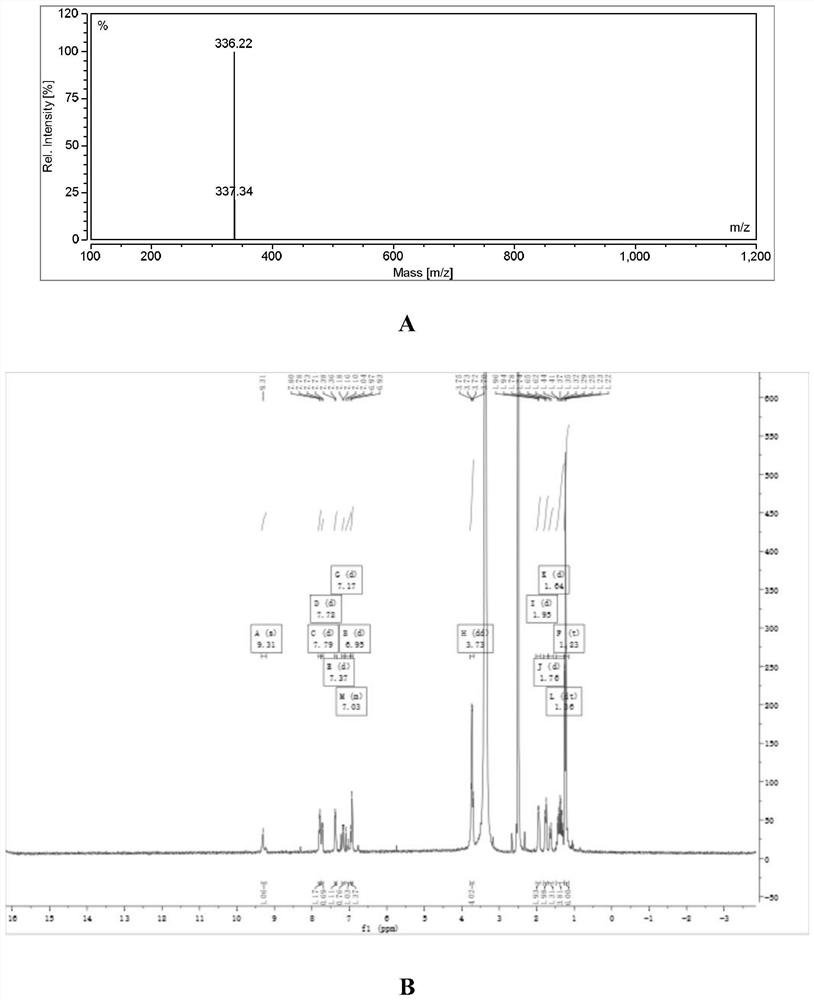
Embodiment 3
[0039] Synthesis of YQN-5 (i.e. compound I-3)
[0040] The synthesis of YQN-5 refers to Example 1. Its mass spectrometry and H NMR spectrum are as image 3 As shown, the specific data are as follows: m / z: 336.22, 1H NMR (400MHz, DMSO) δ9.31 (s, 1H), 7.79 (d, J=9.5Hz, 1H), 7.72 (d, J=9.4Hz, 1H), 7.37(d, J=8.6Hz, 1H), 7.17(d, J=10.0Hz, 1H), 7.11-6.96(m, 1H), 6.95(d, J=16.1Hz, 1H), 3.73( dd, J=14.0, 6.9Hz, 4H), 1.95(d, J=9.9Hz, 2H), 1.76(d, J=13.2Hz, 2H), 1.64(d, J=13.7Hz, 1H), 1.36( dt, J=22.3, 11.5Hz, 4H), 1.23(t, J=7.0Hz, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com