Freeze-dried preparation containing dexrazolone and preparation method of freeze-dried preparation
A technology for dexrazoxane and freeze-dried preparations, which is applied to medical preparations containing active ingredients, medical preparations without active ingredients, freeze-dried transportation, etc. Long drying time and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] prescription:
[0031] Raw materials batch prescription concentration Right Lei Zuosheng 700g 35mg / mL hydrochloric acid 注
180g 0.09mol / L Water for Injection Add water to 20L /
[0032] Note: The concentration of hydrochloric acid is 37wt%, the same below.
[0033] Preparation:
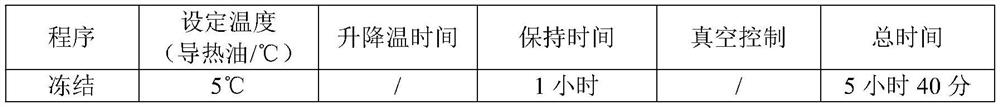
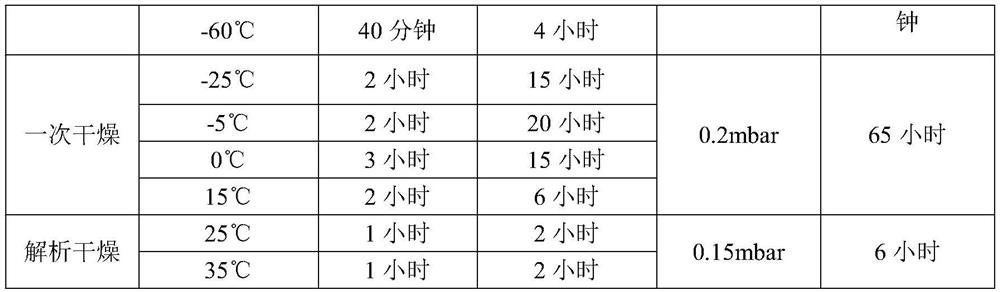
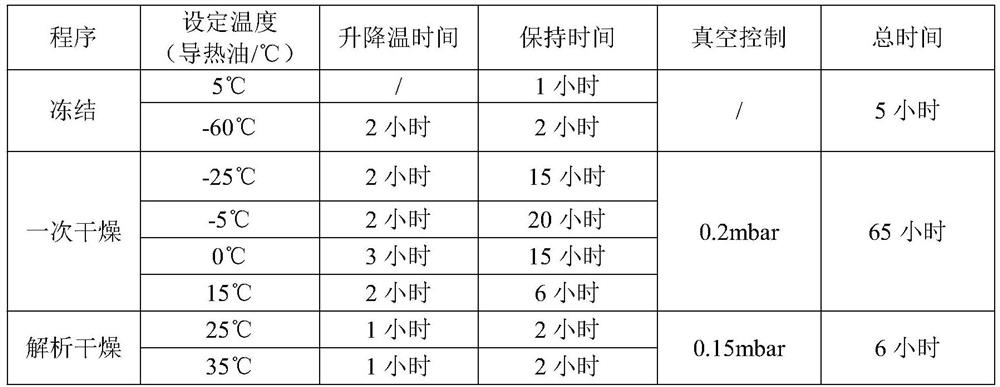
[0034] Lower the temperature of the water for injection to 5°C-10°C, add 18L of water for injection into the liquid mixing tank, add 180g (about 1.8mol) of concentrated hydrochloric acid, stir for 10min, then add the raw material of dexrazoxane, stir for 120min to dissolve it completely Afterwards, take a sample to measure the pH value of 1.8-1.9, then add water for injection (temperature 5-10°C) to the total amount, and stir for 10 minutes. Sterilize and filter through 0.45 μm and 0.22 μm filter elements in turn to the filling room. The filtrate is evenly distributed into 1000 25mL vials, and the liquid level of the lyophilized liquid is 32-35mm. ...
Embodiment 2
[0053] prescription:
[0054] Raw materials batch prescription concentration Right Lei Zuosheng 700g 35mg / mL hydrochloric acid 160g 0.08mol / L Water for Injection Add water to 20L /
[0055] Preparation:
[0056]Lower the temperature of the water for injection to 5°C-10°C, add 18L of water for injection into the liquid mixing tank, add 160g of concentrated hydrochloric acid (about 1.6mol), stir for 10min, then add the raw material of dexrazoxane, stir for 120min to dissolve it completely After that, take a sample to measure the pH value of 1.9-2.0, then add water for injection (temperature 5-10°C) to the total amount, and stir for 10 minutes. Sterilize and filter through 0.45μm and 0.22μm filter elements to the filling room. The filtrate is evenly distributed into 1000 25mL vials, and the liquid level of the lyophilized liquid is 32-35mm.
[0057] The freeze-drying process is the same as in Example 1.
Embodiment 3
[0059] prescription:
[0060] Raw materials batch prescription concentration Right Lei Zuosheng 700g 35mg / mL hydrochloric acid 220g 0.11mol / L Water for Injection Add water to 20L /
[0061] Preparation:
[0062] Lower the temperature of the water for injection to 5°C-10°C, add 18L of water for injection into the liquid mixing tank, add 220g of concentrated hydrochloric acid (about 2.2mol), stir for 10min, then add the raw material of dexrazoxane, stir for 120min to make it completely dissolved After that, take a sample to measure the pH value of 1.6-1.8, then add water for injection (temperature 5-10°C) to the total amount, and stir for 10 minutes. Sterilize and filter through 0.45μm and 0.22μm filter elements to the filling room. The filtrate is evenly distributed into 1000 25mL vials, and the liquid level of the lyophilized liquid is 32-35mm.
[0063] The freeze-drying process is the same as in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| relative humidity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com