Antineoplastic pharmaceutical composition containing piperizine and application of antineoplastic pharmaceutical composition
An anti-tumor drug, the technology of acetazine piperazine, applied in the field of tumor treatment, can solve the problems of reduced treatment effect, severe drug resistance, etc., and achieves the effect of broad market prospect, good treatment effect, and significant killing effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Example 1 In vitro anti-tumor synergy experiment
[0041] (1) Take 786-O cells in the logarithmic growth phase and plant them in a 96-well plate at a certain density (1500-2000 / well);
[0042] (2) Discard the old medium after 24 hours, divide the cells into 5 groups of 1-5 for use, add medium containing different drug concentrations, set 3 duplicate wells for each group, and add sunitinib to group 1 , group 2 was added with piperacetazine, group 3 was added with sunitinib and piperacetazine, group 4 was added with DMSO, and group 5 was used as the blank group; group 1 had an initial concentration of sunitinib of 100 μM, after which each well was sequentially diluted ; The initial concentration of piperazine in group 2 was 100 μM, after which each well was sequentially diluted; in group 3, the initial concentration of sunitinib and piperazine was 100 μM, and then each well was sequentially diluted.
[0043] (3) After culturing for 48 hours, discard the drug solution, ad...
Embodiment 2
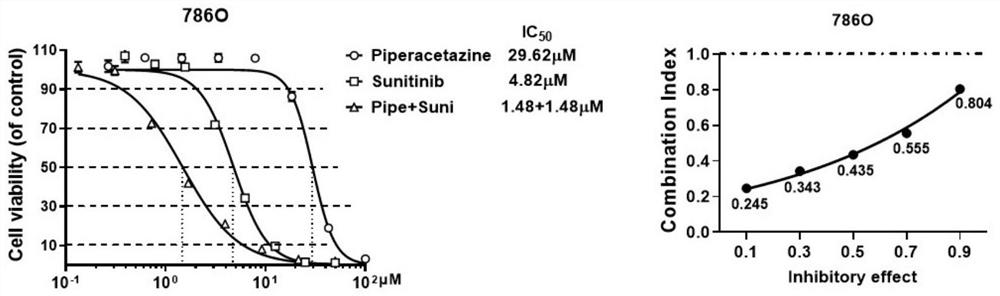
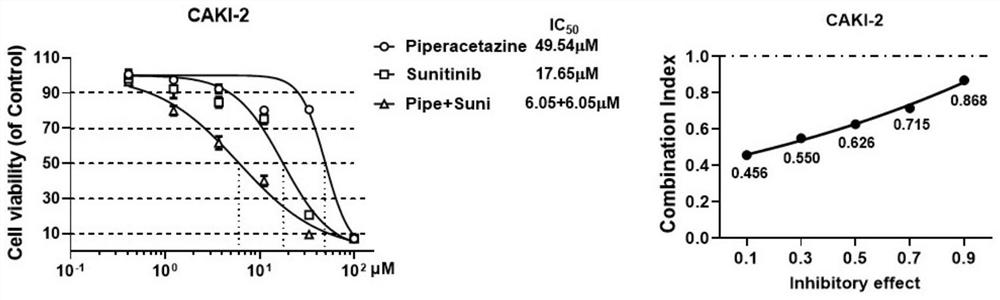
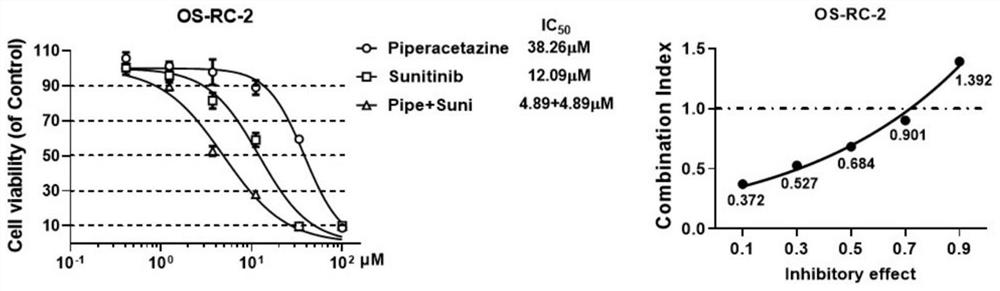
[0054] Example 2 In vitro anti-tumor synergistic compatibility experiment
[0055] (1) 786-O, Caki-2 and OS-RC-2 cells in the logarithmic growth phase were planted in a 96-well plate at a certain density (1500-2000 / well);
[0056] (2) Discard the old medium after 24 hours, add drugs containing different compatible forms to continue the culture, and set 3 duplicate wells for each group;
[0057] (3) After culturing for 48 hours, discard the cell culture medium, add a medium containing 5% CCK-8, incubate at 37°C for 3 hours, and then use a microplate reader to detect the OD value at 450nm.
[0058] Based on the DMSO group, the corresponding synergistic effect evaluation was carried out on the inhibition rate, and the combined drug effect was analyzed by King's formula (Q value):
[0059] Q=E (A+B) / (E A +E B -E A ×E B ), that is, E (A+B) : Inhibition rate of combination drug, E A : Inhibition rate of drug A alone, E B : Inhibition rate of drug B alone. When Q>1.15, it ...
Embodiment 3
[0064] Example 3 In vivo anti-tumor experiment
[0065] Choose Balb / c mice around 5-6 weeks old for in situ tumor growth inhibition experiment in vivo, the specific experimental method is as follows:
[0066] (1) Culture murine renal carcinoma Renca cells in vitro, collect logarithmic phase cells, centrifuge and suspend;
[0067] (2) The cell suspension was injected subcutaneously into 3 Balb / c mice, and after the tumors formed, the tumors were taken out and divided into 5mm 3 Small tumors of left and right size;
[0068] (3) Re-transplant the obtained small tumors under the kidney capsule of several new Balb / c mice;
[0069] (4) After tumor formation, 24 nude mice were randomly divided into 4 groups, numbered 1-4, 6 mice in each group, and administered by intragastric administration; among them, group 1 was given sunitinib-10mg / kg· d, Group 2 was given piperacetylazine-10 mg / kg d, group 3 was given sunitinib-5 mg / kg d+piperacetazine-5 mg / kg d, group 4 was given the same am...
PUM
| Property | Measurement | Unit |
|---|---|---|
| mass | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com