Mirabilite-based phase change material and method for dissolving mirabilite-based phase change material in glycerol system
A technology of phase change material and Glauber's salt, which is applied in the field of Glauber's salt-based phase change materials and its dissolution in glycerin system, can solve the problems of incomplete crystallization and poor cycle stability of Glauber's salt composite phase change materials, and achieve complete crystallization and crystallization volume. Complete, highly crystalline effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
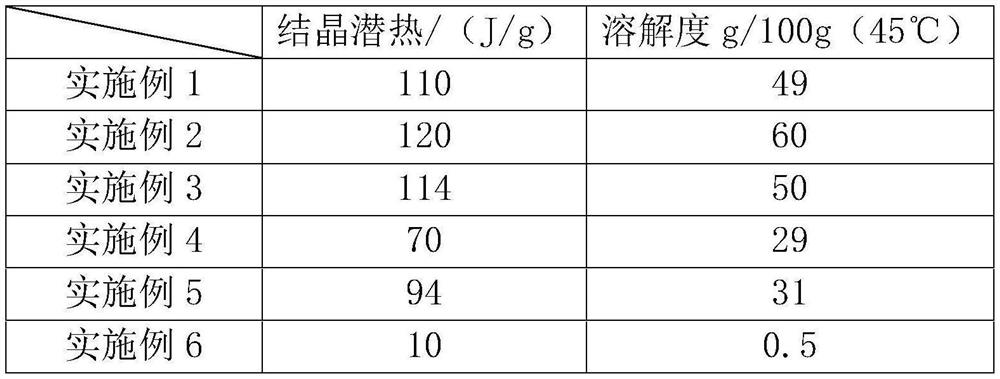
Embodiment 1
[0035] A mirabilite-based phase change material consists of the following raw materials in parts by weight: 49.5 parts of mirabilite, 5.5 parts of sodium carbonate decahydrate, 3 parts of borax, 7 parts of solid sodium hydroxide, 20 parts of glycerin and 80 parts of water. Its dissolving steps are:
[0036] S1, with salt
[0037] Weigh 5.5g of sodium carbonate decahydrate and 49.5g of Glauber's salt at a ratio of 1:9, mix the two, and stir evenly to obtain the solute of Glauber's salt-based compound salt.
[0038] S2, preparation of glycerin solvent
[0039] Weigh 20g of glycerin, 80g of water, and 7g of solid sodium hydroxide, first heat the aqueous solution to 50°C, then add sodium hydroxide to the aqueous solution, stir until the sodium hydroxide is completely dissolved, then add glycerin, and continue stirring at this temperature for 10min , to obtain glycerol solvent.
[0040] S3. Preparation of mirabilite-based phase change material
[0041] Add the mirabilite-based ...
Embodiment 2
[0045] A mirabilite-based phase change material consists of the following raw materials in parts by weight: 90 parts of mirabilite, 10 parts of sodium carbonate decahydrate, 7 parts of borax, 10 parts of solid sodium hydroxide, 25 parts of glycerin and 100 parts of water. Its dissolving steps are:
[0046] S1, with salt
[0047] Weigh 10g of sodium carbonate decahydrate and 90g of Glauber's salt at a ratio of 1:9, mix the two, and stir evenly to obtain the Glauber's salt-based compound salt solute.
[0048] S2, preparation of glycerin solvent
[0049] Weigh 25g of glycerin, 100g of water, and 10g of solid sodium hydroxide, first heat the aqueous solution to 50°C, then add sodium hydroxide to the aqueous solution, stir until the sodium hydroxide is completely dissolved, then add glycerin, and continue stirring at this temperature for 10min , to obtain glycerol solvent.
[0050] S3. Preparation of mirabilite-based phase change material
[0051] Add the mirabilite-based compo...
Embodiment 3
[0055] A mirabilite-based phase change material consists of the following raw materials in parts by weight: 72 parts of mirabilite, 8 parts of sodium carbonate decahydrate, 5 parts of borax, 8 parts of solid sodium hydroxide, 23 parts of glycerin and 92 parts of water. Its dissolving steps are:
[0056] S1, with salt
[0057] Weigh 8g of sodium carbonate decahydrate and 72g of Glauber's salt at a ratio of 1:9, mix the two, and stir evenly to obtain the solute of Glauber's salt.
[0058] S2, preparation of glycerin solvent
[0059] Weigh 23g of glycerin, 92g of water, and 8g of sodium hydroxide solid, first heat the aqueous solution to 50°C, then add sodium hydroxide to the aqueous solution, stir until the sodium hydroxide is completely dissolved, then add glycerin, and continue stirring at this temperature for 10min , to obtain glycerol solvent.
[0060]S3. Preparation of mirabilite-based phase change material
[0061] Add the mirabilite-based compound salt solute obtained...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com