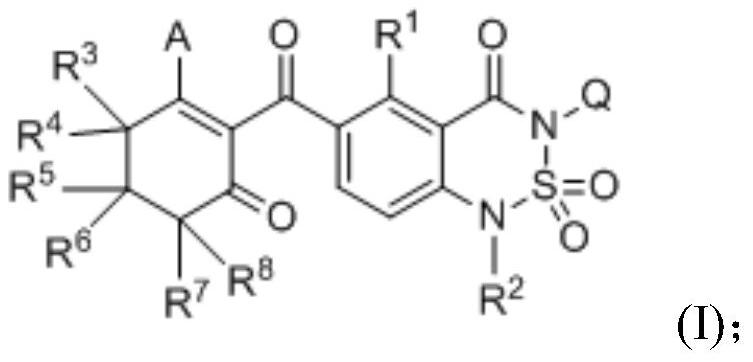
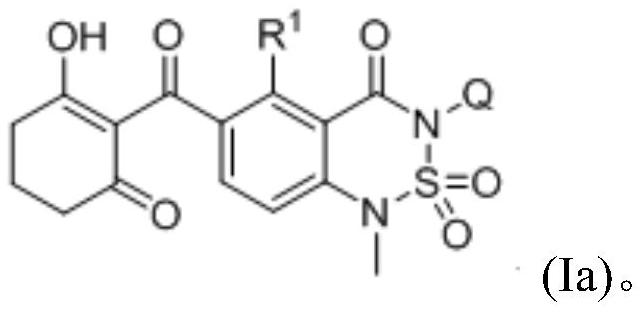
Cyclohexanedione-benzothiadiazine compound and application thereof
A compound, nitrogen oxide technology, applied in application, biocide, organic chemistry, etc., can solve the problems of insufficient herbicidal effect, no weed plants, narrow weed plant spectrum, etc., to achieve good control effect and crop safety. , the effect of drug effect is fast
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0151] Example 1: Compound 3-(2,6-dimethylphenyl)-6-(2-hydroxyl-6-oxocyclohex-1-enecarbonyl)-1-methyl-1H-benzo[c Synthesis of ][1,2,6]thiadiazin-4(3H)-one 2,2-dioxide
[0152]
[0153] Step 1: Synthesis of intermediate 4-nitroisophthalic acid
[0154]
[0155] Potassium hydroxide (12.39g, 662mmoL), water (400mL) and 2-nitro-5-methylbenzoic acid (40g, 220mmoL) were added to a 1-liter reaction flask, and heated to 90°C. After the solid was completely dissolved, Add potassium permanganate (104.69g, 662mmoL) in batches, react at 90°C for 3 hours, stop heating, filter the system while it is hot, rinse the filter residue with water (50mL×2), and adjust the pH to 1 after the mother liquor drops to room temperature , and then stirred at 0° C. for 30 minutes, filtered, and dried the solid to obtain 43 g of a white solid with a yield of 92%.
[0156] MS(ES-API,pos.ion)m / z:209.9[M-H] - .
[0157] Step 2: Synthesis of intermediate dimethyl 4-nitroisophthalate
[0158]
[015...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com