Selective histamine h3 antagonist acid addition salts and process for the preparation thereof
A citrate, acetone dicitrate technology, applied in the direction of organic active ingredients, organic chemical methods, medical preparations containing active ingredients, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
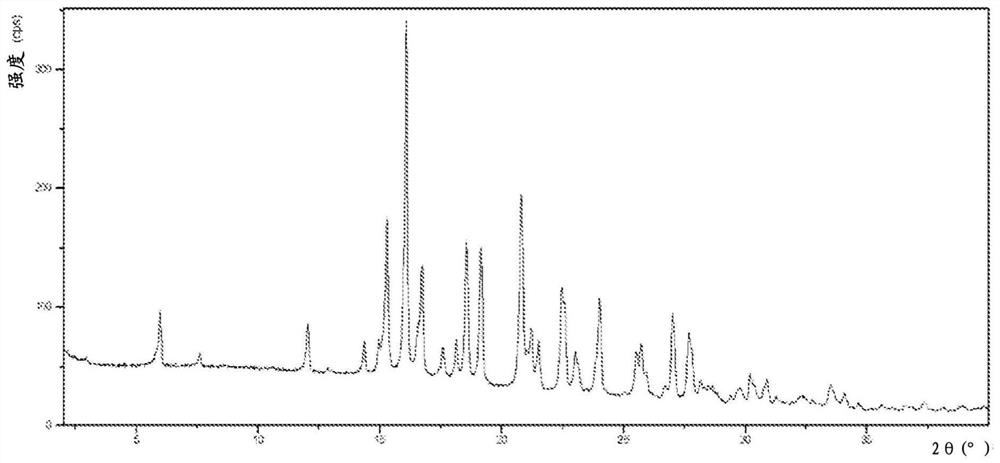
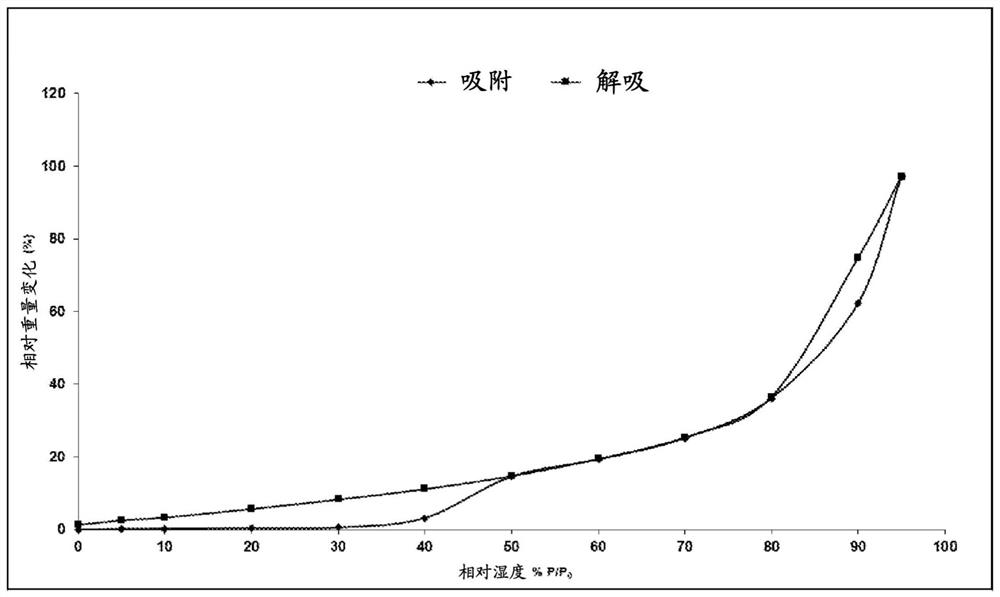
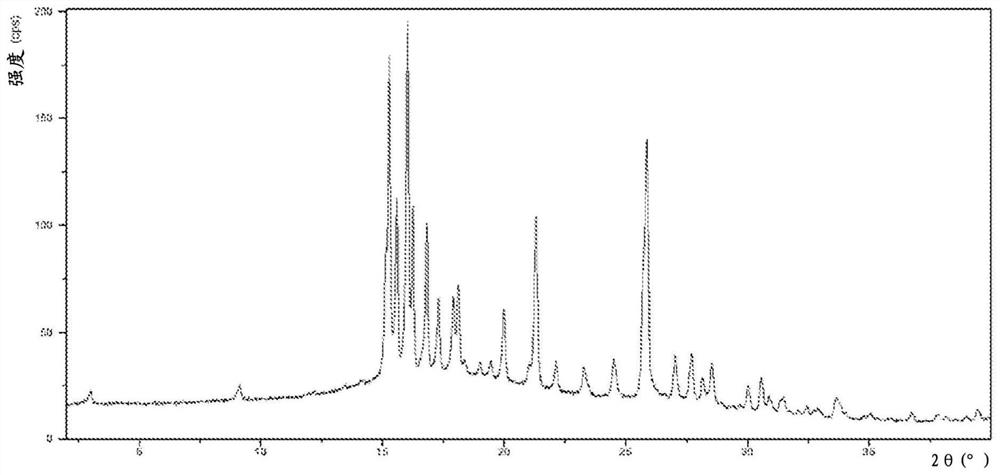
Image
Examples
Embodiment 1
[0146] 1-[4-(4-{3-[(2R)-2-Methyl-pyrrolidin-1-yl]-propoxy}-phenoxy)-piperidin-1-yl]-ethanone
[0147] 40 g of 1-[4-(4-{3-[(2R)-2-methyl-pyrrolidin-1-yl]-propoxy}-phenoxy prepared according to Example 11 of WO 2014 / 136075 )-piperidin-1-yl]-ethanone hydrochloride was dissolved in 480 mL of dichloromethane at 0-5 °C, and then 168 mL of 1M aqueous NaOH was added. After stirring for 10 minutes, the aqueous and organic phases were separated, and the organic phase was washed twice with 120 mL of deionized water, dried with 25 g of sodium sulfate and filtered. The solution was concentrated in vacuo to an oil. Evaporation residue: 32.8 g oil.
Embodiment 2
[0149] 1-[4-(4-{3-[(2R)-2-Methyl-pyrrolidin-1-yl]-propoxy}-phenoxy)-piperidin-1-yl]-ethanone Dihydrochloride
[0150] At room temperature, 2.0 g (5.55 mmol) of 1-[4-(4-{3-[(2R)-2-methyl-pyrrolidin-1-yl]-propoxy}-phenoxy)-piper Pyridin-1-yl]-ethanone was dissolved in 20 mL of acetone. The reaction mixture was cooled to 0 to 5 °C and 0.8 mL > 37% hydrochloric acid solution was added dropwise. After stirring at 0 to 5°C for 30 minutes, the crystals were filtered, covered with 1.5 mL of cold acetone, and dried at room temperature. White crystalline substance. Yield: 1.7g.
Embodiment 3
[0152] 1-[4-(4-{3-[(2R)-2-Methyl-pyrrolidin-1-yl]-propoxy}-phenoxy)-piperidin-1-yl]-ethanone Dihydrochloride
[0153] At room temperature, 0.548 g of 1-[4-(4-{3-[(2R)-2-methyl-pyrrolidin-1-yl]-propoxy}-phenoxy)-piperidine-1 -yl]-ethanone was dissolved in 1.1 mL of isopropanol. At room temperature, 0.391 g of 30% isopropanol hydrochloride was added dropwise to the alkaline solution. The precipitated slurry was filtered, then vacuum dried at 40°C under nitrogen for 2 hours. Yield: 0.42g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com