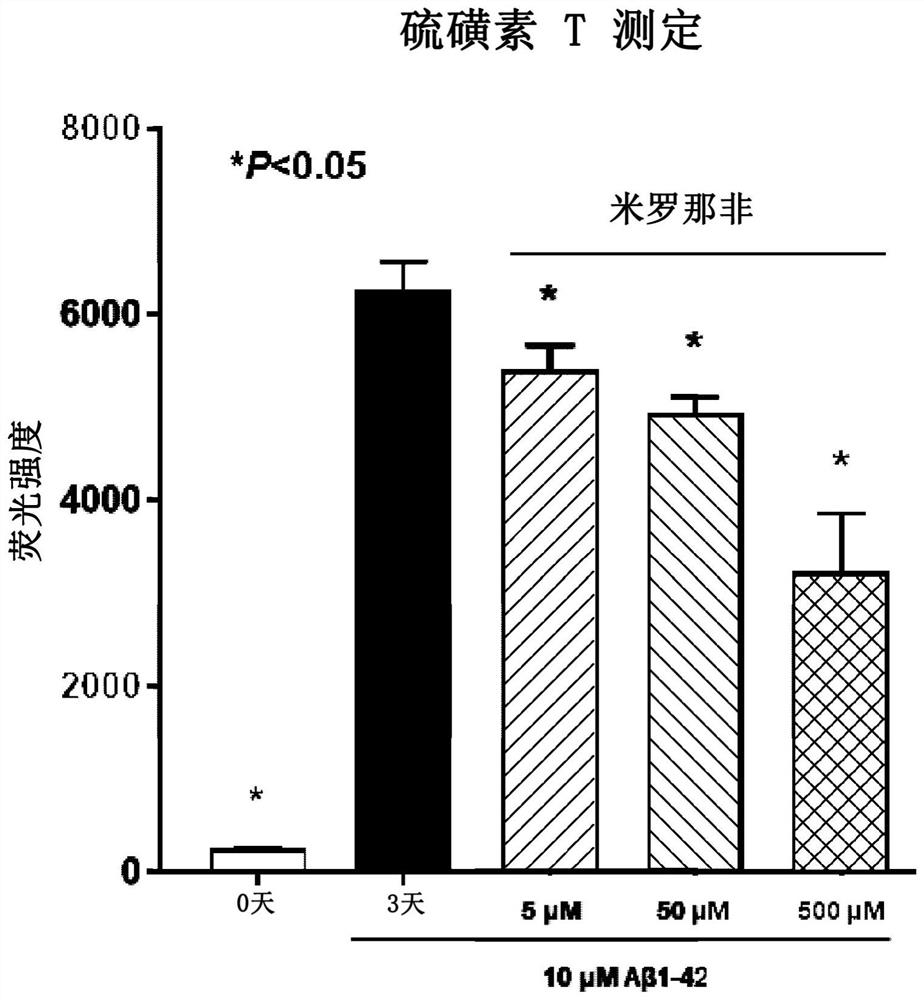
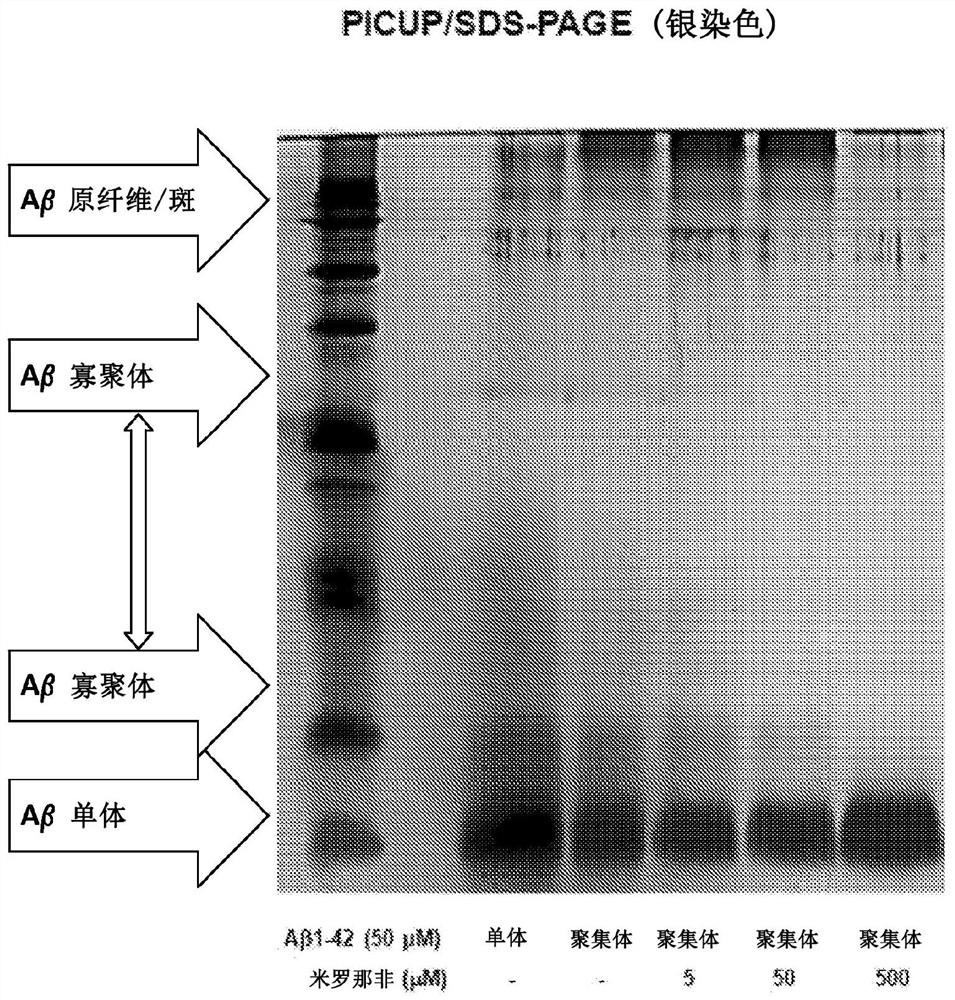
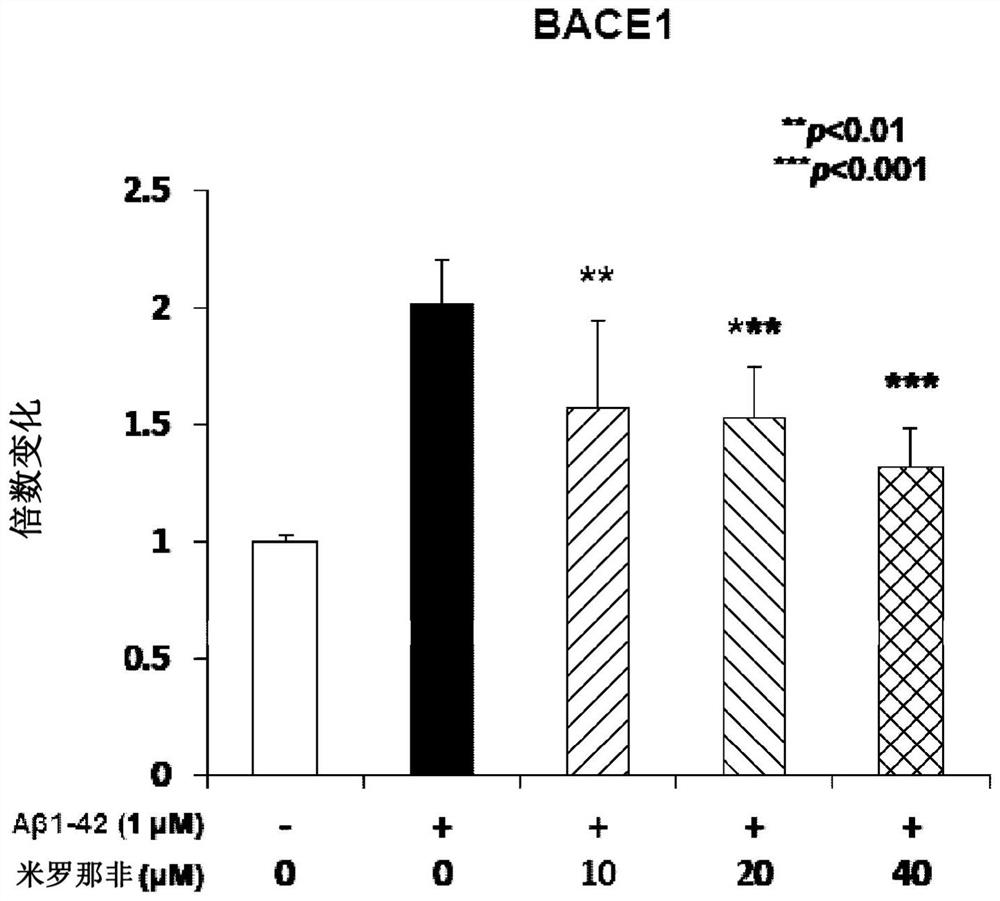
Compositions and methods for reducing amyloid beta formation on and composition therefore
A composition and compound technology, which is applied in the direction of drug combination, active ingredient of heterocyclic compound, pharmaceutical formulation, etc., can solve problems such as the inability to successfully develop therapeutic agents for dementia
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Embodiment 1. The selectivity test (IC of the composition of the present invention to PDE5) 50 , inhibitory concentration 50)
Embodiment 1-1
[0104] Embodiment 1-1. is used for the test method of PDE5 selectivity
[0105] In order to test the selective inhibitory activity (IC of the composition of the present invention) to PDE5 (phosphodiesterase 5) 50 , inhibitory concentration 50), the test was performed by MDS Pharma Services, analysis agency CRO (analysis agency CRO) and ScottishBiomedical. Results were measured using the PDE SPA assay kit (Amersham Pharmacia Biotech). MDS Pharma Services analyzed PDE1 (bovine heart); PDE 2, 3, 5 (human platelets); PDE4 (human U937 cells); and PDE6 (bovine rod cells) and Scottish Biomedical analyzed PDE 5, 7-11 (recombinant human enzyme). Add test samples (100 μL each) to PDE family proteins (10 μl), [ 3 H]-cGMP (5Ci / mL), bovine serum albumin (0.5mg / mL) and MgCl 2 (5mM) in a mixture in Tris-HCl buffer (15mM, pH 7.5). The reaction begins with the addition of PDE family proteins. Each sample was stored in a 30° C. water bath for 30 minutes, and then SPA beads (PerkinElmer) (...
Embodiment 1-2
[0106] Example 1-2. Results of PDE5 selectivity tests for compositions of the present invention
[0107] Based on the inhibitory activity of the composition of the present invention on 11 PDE families (MDS Pharma Services and Scotish Biomedical), the IC of the composition of the present invention on PDE5 50 was 0.338 nM (MDS Pharma Services) and was 30-376,471 times selective over the remaining 10 PDE family proteins (Table 1).
[0108] Table 1. Selectivity results for PDE families of the invention
[0109]
[0110] a MDS Pharma Services; PDE1 (bovine heart); PDE 2, 3, 5 (human platelets); PDE4 (human U937 cells); PDE6 (bovine rod cells).
[0111] b Scottish Biomedical; PDE 5, 7-11 (recombinant human enzyme); NI = no inhibition observed.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com