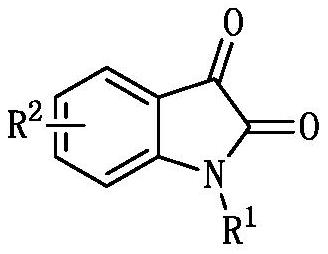
Synthesis method of (1,1-dicyano-4-oxygen)-hexyl-3-oxindole phosphonite diethyl and its derivatives
A technology of diethyl indole phosphinate and synthesis method is applied in the field of synthesis of diethyl-hexyl-3-oxyindole phosphinate and derivatives thereof, and achieves high reaction yield, simple steps and high reaction efficiency. short time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
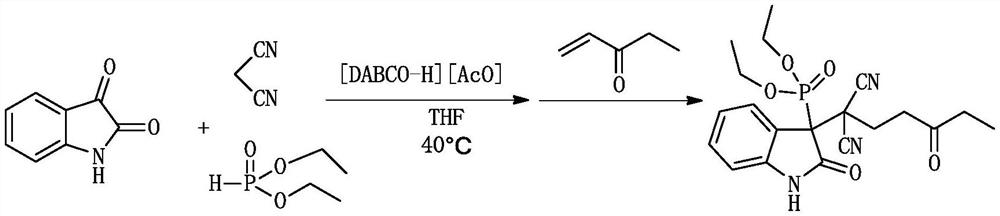
[0027] A kind of (1,1-dicyano-4-oxo)-hexyl-3-oxyindole phosphonite and its derivative (1,1-dicyano-4-oxo)-hexyl-3 -The synthetic method of oxyindole phosphinate, comprising:
[0028]
[0029] 1.0 mmol (0.147 g) isatin, 1.0 mmol (0.066 g) malononitrile, 1.0 mmol (0.138 g) diethyl phosphite and 0.2 mmol ionic liquid catalyst 1,4-diazabicyclo[2.2.2] were successively mixed Octane acetate was added to a 10ml dry round-bottomed flask, then 1ml of tetrahydrofuran was added as a solvent, heated and stirred at 40°C for 30min, and then 1.0mmol (0.084g) of 1-penten-3-one was added to continue the reaction at 40°C, The reaction was monitored by TLC. After the reaction was completed (1.5h), 10ml of water was added. After cooling, 10ml of ethyl acetate was added for extraction 3 times. The organic phase was dried over anhydrous sodium sulfate, filtered and evaporated, and the crude product was separated by column chromatography. Purify, adopt petroleum ether and ethyl acetate as eluent...
Embodiment 2
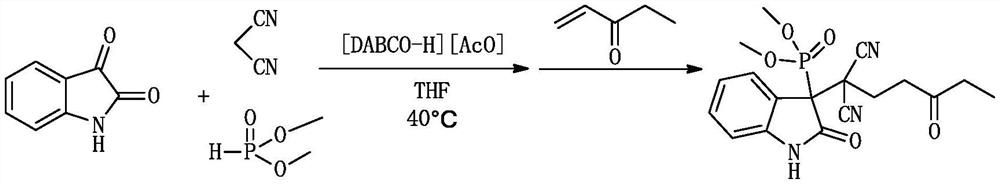
[0034] A kind of (1,1-dicyano-4-oxo)-hexyl-3-oxyindole phosphonite and its derivative (1,1-dicyano-4-oxo)-hexyl-3 -The synthetic method of dimethyl oxyindole phosphinate, comprising:
[0035]
[0036]1.0 mmol (0.147 g) isatin, 1.0 mmol (0.066 g) malononitrile, 1.0 mmol (0.110 g) dimethyl phosphite and 0.2 mmol ionic liquid catalyst 1,4-diazabicyclo[2.2.2] Octane acetate was added to a 10ml dry round-bottomed flask, then 1ml of tetrahydrofuran was added as a solvent, heated and stirred at 40°C for 30min, and then 1.0mmol (0.084g) of 1-penten-3-one was added to continue the reaction at 40°C, The reaction was monitored by TLC. After the reaction was completed (36h), 10ml of water was added. After cooling, 10ml of ethyl acetate was added for extraction 3 times. The organic phase was dried over anhydrous sodium sulfate, filtered and evaporated, and the crude product was separated and purified by column chromatography. , using petroleum ether and ethyl acetate as eluent (in part...
Embodiment 3
[0041] A kind of (1,1-dicyano-4-oxo)-hexyl-3-oxyindole phosphonite and its derivative (1,1-dicyano-4-oxo)-hexyl-3 -The synthetic method of diphenyl oxyindole phosphinate, comprising:
[0042]
[0043] 1.0 mmol (0.147 g) isatin, 1.0 mmol (0.066 g) malononitrile, 1.0 mmol (0.110 g) dimethyl phosphite and 0.2 mmol ionic liquid catalyst 1,4-diazabicyclo[2.2.2] Octane acetate was added to a 10ml dry round-bottomed flask, then 1ml of tetrahydrofuran was added as a solvent, heated and stirred at 40°C for 30min, and then 1.0mmol (0.084g) of 1-penten-3-one was added to continue the reaction at 40°C, The reaction was monitored by TLC. After the reaction was completed (12h), 10 ml of water was added. After cooling, 10 ml of ethyl acetate was added for extraction three times. The organic phase was dried over anhydrous sodium sulfate, filtered and evaporated, and the crude product was separated and purified by column chromatography. , using petroleum ether and ethyl acetate as eluent (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com