Application of MLL-menin inhibitor composition in preparation of anti-hepatoma drug
A technology of inhibitors and compositions, applied in anti-tumor drugs, drug combinations, pharmaceutical formulations, etc., can solve problems such as unsatisfactory curative effect of hepatocellular carcinoma, and achieve the effects of enhancing binding effect, reducing expression level, and increasing sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
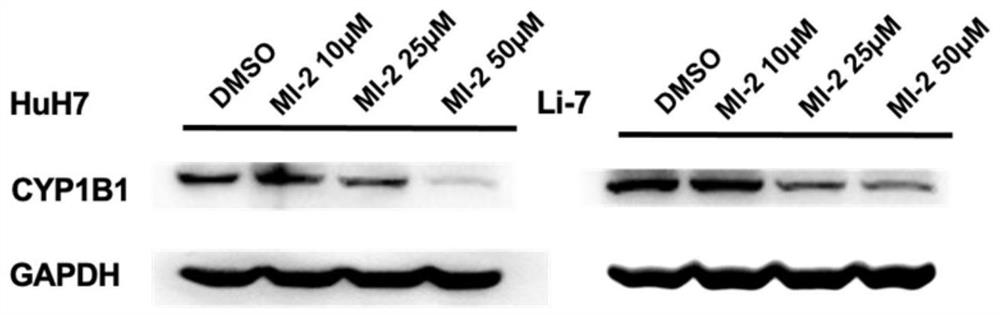
[0025] The present invention finds that the MLL-menin inhibitor MI-2 composition can down-regulate the protein level of CYP1B1 in the hepatocellular carcinoma cell lines Huh7 and Li-7.
[0026] Reagents and materials:
[0027] The liver cancer cell lines HuH-7 and Li-7 were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences. HuH7 was cultured in DMEM medium (CORNING, product number 10-013-CV) containing 10% GBICO fetal bovine serum, Li-7 was cultured in RPMI-1640 medium (CORNING, product number 10-013-CV, adding Sodium Pyruvate 0.11 g / L), and containing 10% fetal bovine serum; culture conditions are 37 ℃, 5% CO2. MI-2 was purchased from TargetMol (Cat. No. T2649).
[0028] experimental method:
[0029] Treat Huh7 and Li-7 cells with DMSO (dimethyl sulfoxide) and MI-2 (10 μmol / L, 25 μmol / L, 50 μmol / L) respectively, extract cell sample protein, pre-cool RIPA lysate on ice, and use immediately Before adding protease inhibitor PMSF at a final concentratio...
Embodiment 2
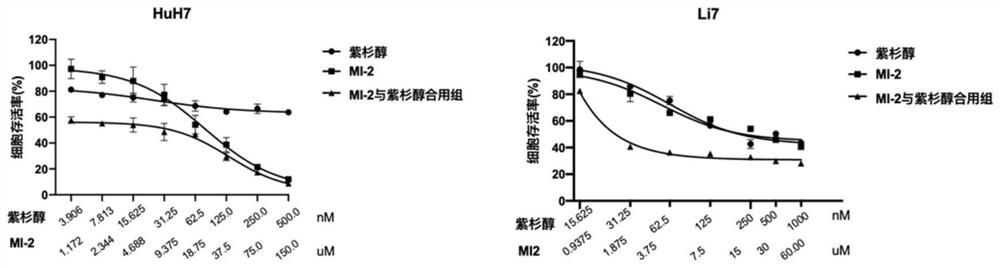
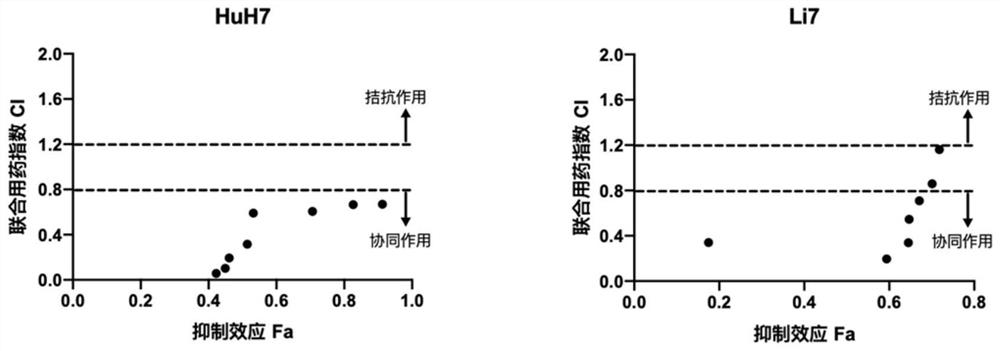
[0033] The present invention found that the combination drug MI-2 and paclitaxel can synergistically enhance the toxicity of paclitaxel to hepatocellular carcinoma cell lines.
[0034] Reagents and materials:
[0035] Paclitaxel was purchased from Aladdin (Product No. P106869), and other reagent materials were the same as in Example 1.
[0036] experimental method:
[0037] Preparation of MI-2, paclitaxel stock solution and working solution:
[0038] Using DMSO as solvent, dilute MI-2 to 500x stock solution, paclitaxel to 1000x stock solution, and store at -20°C.
[0039] The working solution is prepared immediately before use, and 10 μL working solution / 100 μL culture system / well is added to each well when dosing:
[0040] MI-2 working solution: 2μL stock solution + 98μL medium → 100μL working solution (10x)
[0041] Paclitaxel working solution: 1 μL stock solution + 99 μL medium → 100 μL working solution (10x)
[0042] Cell culture and dosing:
[0043]HuH7 (5000cells / w...
Embodiment 3
[0058] The present invention finds that the combined drug MI-2 and paclitaxel can promote microscopic protein aggregation and increase the toxicity of paclitaxel in the mouse model.
[0059] Reagents and materials:
[0060] Female Balb / C nude mice were purchased from Shanghai Experimental Animal Research Center and bred in the Experimental Animal Center of Zhejiang University. All the other reagent materials are the same as in Example 1.
[0061] experimental method:
[0062] A mouse model of hepatocellular carcinoma was constructed, and Balb / C nude mice aged 4 to 6 weeks were used as experimental objects, and divided into four groups: solvent control group, MI-2 group, paclitaxel group and paclitaxel and MI-2 combined group, divided into 5 groups Only. Will 1x10 7 A large number of Li-7 or Huh7 cells were inoculated in the axilla of nude mice. For PDX models, the tumor tissues of hepatocellular carcinoma patients with the size of mung beans were inoculated in the axilla o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com