Preparation method of 2, 6-di-tert-butyl-4-bromoanisole
A technology of bromoanisole and di-tert-butyl, which is applied in the field of preparation of 2,6-di-tert-butyl-4-bromoanisole, can solve the problems of low production efficiency, high cost, unfavorable environmental protection, etc., and achieve High production efficiency, low production cost, and environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
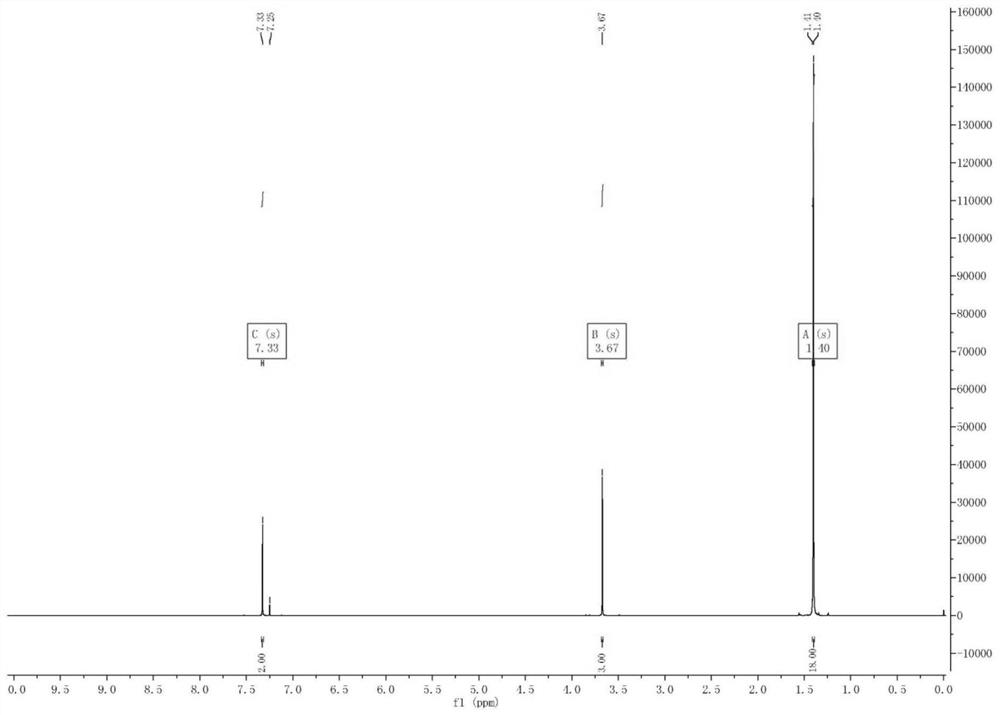
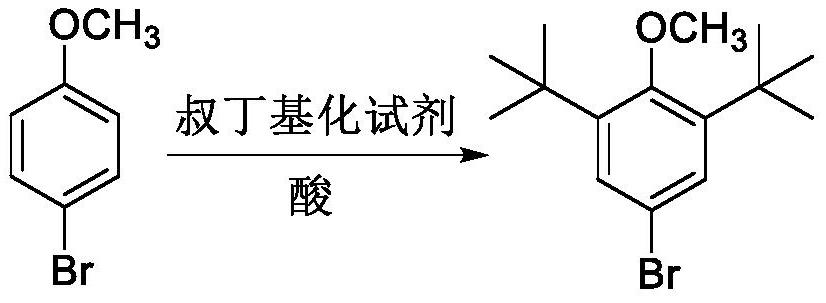
[0021] The invention provides a preparation method of 2,6-di-tert-butyl-4-bromoanisole, comprising: adding a tert-butylating reagent to 4-bromoanisole under the catalysis of an acid for reaction to obtain 2 ,6-Di-tert-butyl-4-bromoanisole.
[0022] Above-mentioned preparation method can be represented by following reaction formula:
[0023]
[0024] The above reaction is essentially a Friedel-Crafts alkylation reaction. First, under the catalysis of an acid, the tert-butylating reagent forms a tert-butyl carbocation intermediate, and the tert-butyl carbocation intermediate acts as an electrophile and a raw material 4-Bromoanisole undergoes an electrophilic substitution reaction, and both methoxy and bromine are ortho-para positioning groups, but the activating ability of methoxy to benzene ring is stronger than that of bromine to benzene ring, so the two The tert-butyl group is generated at the ortho position of the methoxy group, thus obtaining 2,6-di-tert-butyl-4-bromoan...
Embodiment 1
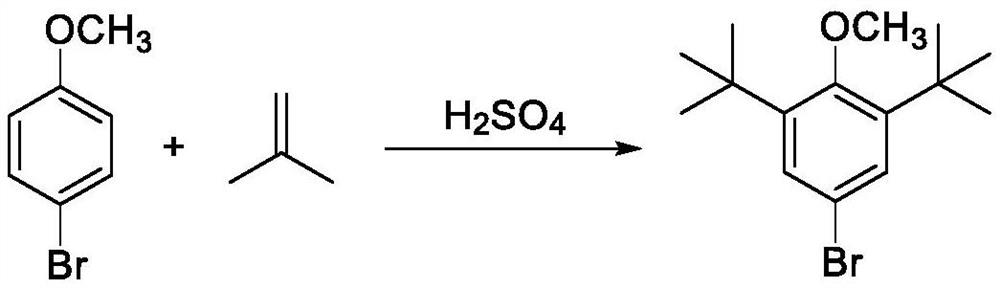
[0055] The preparation process of the 2,6-di-tert-butyl-4-bromoanisole of the present embodiment can be represented by the following reaction formula:
[0056]
[0057] Concrete preparation method comprises the following steps:
[0058] 1. At room temperature, add 186g (1mol) of 4-bromoanisole and 5g (concentration: 98%, 0.05mol) of concentrated sulfuric acid to the flask and stir, then feed isobutylene gas into the flask to make the air in the flask completely Replacement, after the replacement is completed, the temperature of the reaction system is raised to 55-65°C under stirring, and 168 g (3 mol) of isobutylene gas is continuously introduced into the reaction system after the temperature rise is completed, and the reaction is continued at 55-65°C for 2 hours;
[0059] After reacting for 2 hours, sampling was carried out, and it was detected by HPLC that the 4-bromoanisole raw material had reacted completely, and it was judged that the reaction ended;
[0060] 2. Add 1...
Embodiment 2
[0065] The preparation process of the 2,6-di-tert-butyl-4-bromoanisole of the present embodiment can be represented by the following reaction formula:
[0066]
[0067] Concrete preparation method comprises the following steps:
[0068] 1. At room temperature, first add 186g (1mol) of 4-bromoanisole and 200g (concentration: 98%, 2mol) of concentrated sulfuric acid into the flask, and stir and mix evenly, then heat up the reaction system to 35-45°C, and At this temperature, 185g (2.5mol) of tert-butanol was added dropwise to the mixed solution of 4-bromoanisole and concentrated sulfuric acid, and after the dropwise addition was completed, the reaction was continued at 35-45°C for 2 hours;
[0069] After reacting for 2 hours, sampling was carried out, and it was detected by HPLC that the 4-bromoanisole raw material had reacted completely, and it was judged that the reaction ended;
[0070] 2. Add 100g of deionized water to the reaction system at 55-65°C, stir for 10 minutes,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com