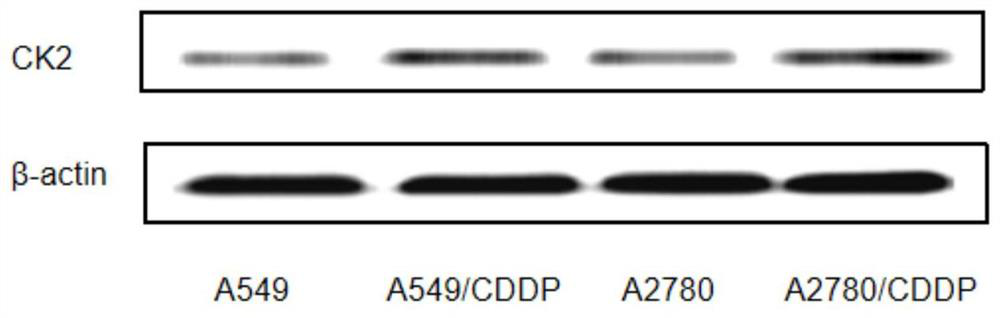
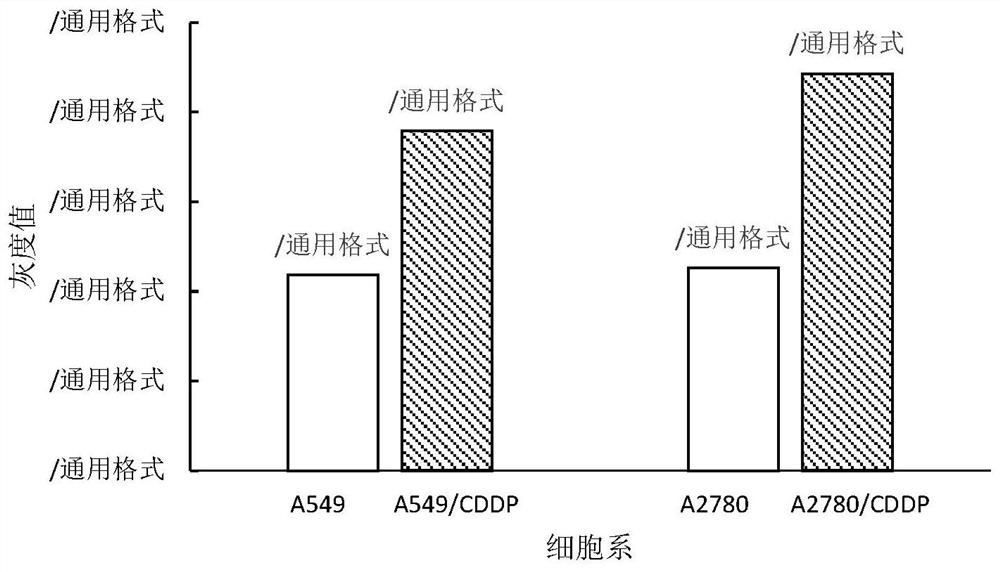
Anti-tumor compound capable of overcoming cis-platinum drug resistance and preparation and application thereof
A compound, anti-tumor technology, applied in the field of anti-tumor compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment one: the preparation of compound 1
[0057] Suspend equimolar amounts of compound 3 and compound 4 (2mmol) in 30mL of anhydrous methanol in a 50mL reaction flask, add 2-3 drops of acetic acid, and react at 30-60°C in the dark for 48-72h. TLC monitors the compound 3 complete responses. After cooling and filtering, the filter cake was washed with 30 mL (3×10 mL) of methanol to obtain a yellow solid product with a yield of 96.4%.
[0058] 1 H NMR (600MHz, DMSO-d6) δ11.15(s, 1H), 10.19(s, 1H), 9.67(s, 1H), 8.99(d, J=4.2Hz, 1H), 8.86(d, J= 7.7Hz, 1H), 8.58(d, J=3.8Hz, 1H), 8.26(s, 1H), 8.23(s, 1H), 8.14(d, J=7.2Hz, 1H), 7.93(d, J= 7.3Hz, 1H), 7.45(t, J=7.5Hz, 1H), 7.14(d, J=6.9Hz, 1H), 4.26(s, 6H), 3.74(s, 4H)ppm. 13 C NMR(150MHz,DMSO-d6)δ176.45,176.23,163.28,159.72,150.55,148.18,147.80,143.63,142.33,135.12,133.28,130.62,127.50,126.91,124.23,123.84,122.80,122.69,121.73,120.64,119.68 ,116.83,56.40,49.60,46.96ppm.HR-MS(m / z)(ESI):calcd for C 25 h 23 ClN 7 o ...
Embodiment 2
[0059] Embodiment two: the preparation of compound 2
[0060] Compound 3 and compound 5 were prepared according to the method shown in Example 1 to obtain a yellow solid product with a yield of 95.2%.
[0061] 1 H NMR (600MHz, DMSO-d6) δ10.18(s, 1H), 10.09(s, 1H), 9.68(s, 1H), 8.99(d, J=5.5Hz, 1H), 8.84(d, J= 8.4Hz,1H),8.59(d,J=5.4Hz,1H),8.35(s,1H),8.23(s,1H),8.09(d,J=8.1Hz,1H),7.93(d,J= 8.2Hz, 1H), 7.45(t, J=8.0Hz, 1H), 7.15(d, J=7.7Hz, 1H), 6.01(d, J=8.0Hz, 2H), 5.30(m, 2H), 3.70 -3.79(m,4H),2.07(s,2H),1.83(d,J=11.9Hz,2H),1.44-1.46(m,2H),1.22(d,J=8.8Hz,2H),1.02( t,J=9.5Hz,2H)ppm. 13C NMR(150MHz,DMSO-d6)δ206.07,176.03,165.83,150.48,148.15,147.72,143.76,142.39,134.71,133.28,130.54,127.52,126.26,124.19,123.01,122.86,122.62,121.48,120.56,119.57,116.82 ,62.57,56.35,46.86,31.96,24.50ppm.HR-MS(m / z)(ESI):calcd forC 31 h 31 ClN 7 o 5 Pt[M+H] + :811.1717; found: 811.1158.
Embodiment 3
[0062] Embodiment three: the preparation of compound 3
[0063] Compound 6 (1.09g, 3mmol) was dissolved in 20mL of methanol in a 50mL single-necked bottle, and hydrazine hydrate (6-30mmol) was slowly added dropwise at room temperature, and heated to reflux for 24-72h until the reaction was complete. After cooling, a large amount of solid precipitated out, and was suction filtered, and the filter cake was washed with 3×10 mL ice methanol to obtain 1.05 g of a bright yellow solid product, with a yield of 97.1%. 1 H NMR (600MHz, DMSO-d6) δ10.14(s, 1H), 10.08(s, 1H), 9.63(s, 1H), 8.95(d, J=6.0Hz, 1H), 8.80(d, J= 12.0Hz, 1H), 8.55(d, J=6.0Hz, 1H), 8.33(t, J=1.8Hz, 1H), 8.21(d, J=1.6Hz, 1H), 8.07(d, J=6.0Hz ,1H),7.91(dd,J=12.0,1.6Hz,1H),7.42(t,J=6.0Hz,1H),7.12(dd,J=8.0,1.4Hz,1H),4.63(s,2H) ppm. 13 C NMR(150MHz,DMSO-d6)δ165.77,150.47,148.13,147.69,143.76,142.40,134.73,133.35,130.52,127.52,126.26,124.17,123.00,122.83,122.60,121.47,120.57,119.56,116.79ppm.HR- MS(m / z)(ESI):calcd for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com