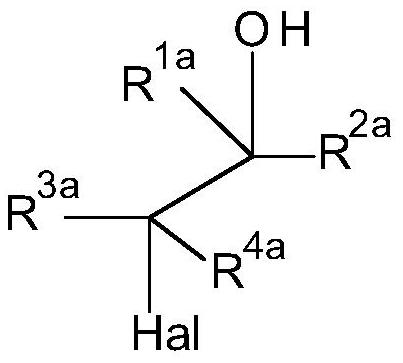
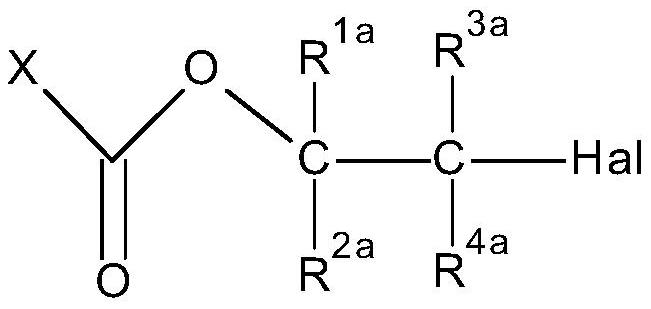
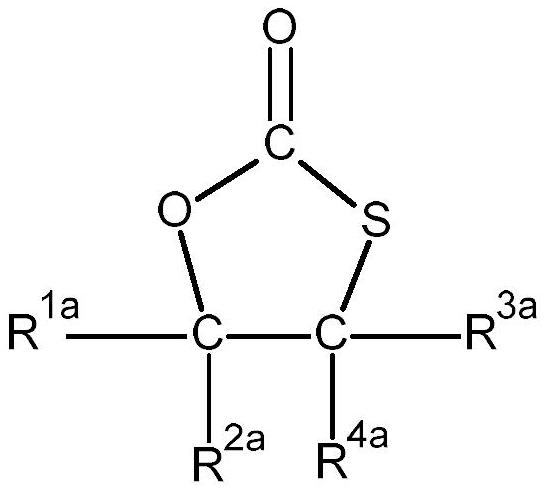
Method for preparation of thiocarbonates
A monothiocarbonate, carbocyclic technology, applied in organic chemistry and other directions, can solve problems such as performance problems, low availability of thiocarbonates, and low availability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0179] GC analysis: Agilent Technologies 7890 A Network GC System
[0180] Column: DB1 (Agilent) 30m, Film thickness 1μm;
[0181] Carrier gas He; flow rate 1.0mL / min; split ratio: 50:1
[0182] T program: 50-300°C, heating rate 10°C / min; 30min isothermal
[0183] Temperature (injection system) 250°C
[0184] Preparation of 2-chloroethyl chloroformate, process step b)
[0185]
[0186] The synthesis was performed in a 1 L stirred tank reactor equipped with two condensers (-30°C and -78°C (dry ice)), phosgene dip tube, thermometer and dropping funnel. Phosgene (46.0 g, 0.5 mol) was introduced into the empty reactor at a temperature of 10°C until phosgene reflux was observed. Then 2-chloroethanol (161 g, 2.00 mol) was added via dropping funnel over 2 hours. The internal temperature recorded during this time can vary within 10-14 °C. Additional phosgene (404 g, 4.0 mol) was added simultaneously during the addition of 2-chloroethanol to ensure phosgene reflux throughout...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com