Automatic sample workflow for lc-ms based hba1c measurement on intact protein level
A sample, complete technology in the field of automated sample workflows for LC-MS based HbA1c measurements on intact protein levels, addressing the issue of not providing satisfactory and reproducible results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0062] A first aspect of the invention relates to a method for determining the intact molecule of glycated hemoglobin A (HbA1c) in a sample, the method comprising:
[0063] (a) providing a sample comprising hemoglobin A (HbA) molecules, particularly intact hemoglobin A (HbA) molecules;
[0064] (b) enriching said sample for hemoglobin A molecules, in particular alpha chain and / or beta chain molecules; and
[0065] (c) Subjecting a sample comprising intact hemoglobin A molecules, in particular alpha and / or beta chain molecules, to analysis by mass spectrometry (MS), wherein the amount or concentration of HbA1c in the sample is determined.
[0066] Thereby, the amount or concentration of HbA1c in the sample can be determined, in particular the relative amount of HbA1c, ie the ratio of glycated HbA1c molecules to non-glycated HbA0 molecules or to total HbA molecules. When repeatedly determining the amount of HbA1c, the ratio of HbAlc / HbA or the ratio of HbAlc / HbA0 in a given sam...
example 1
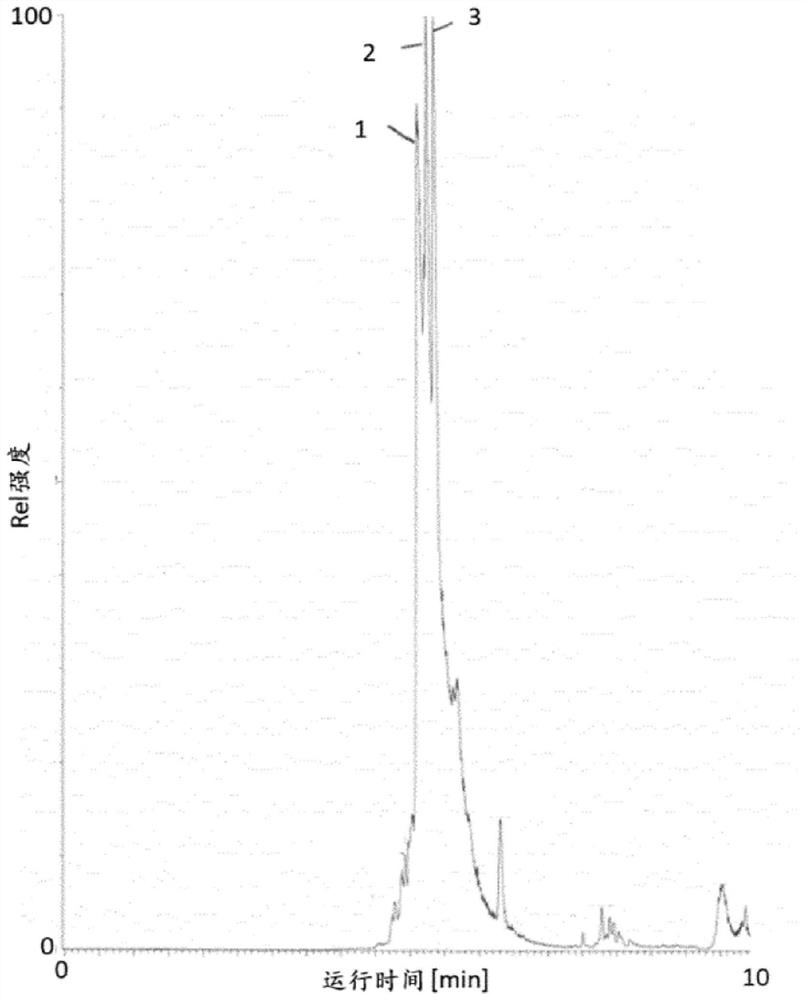
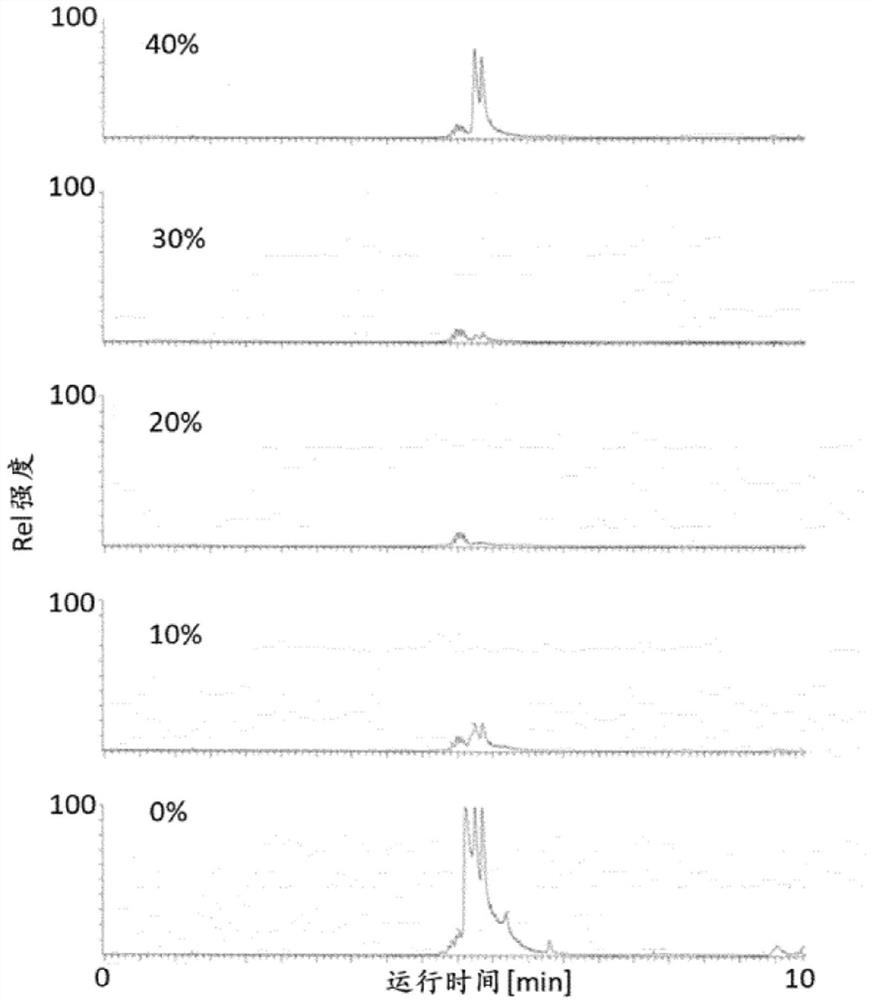
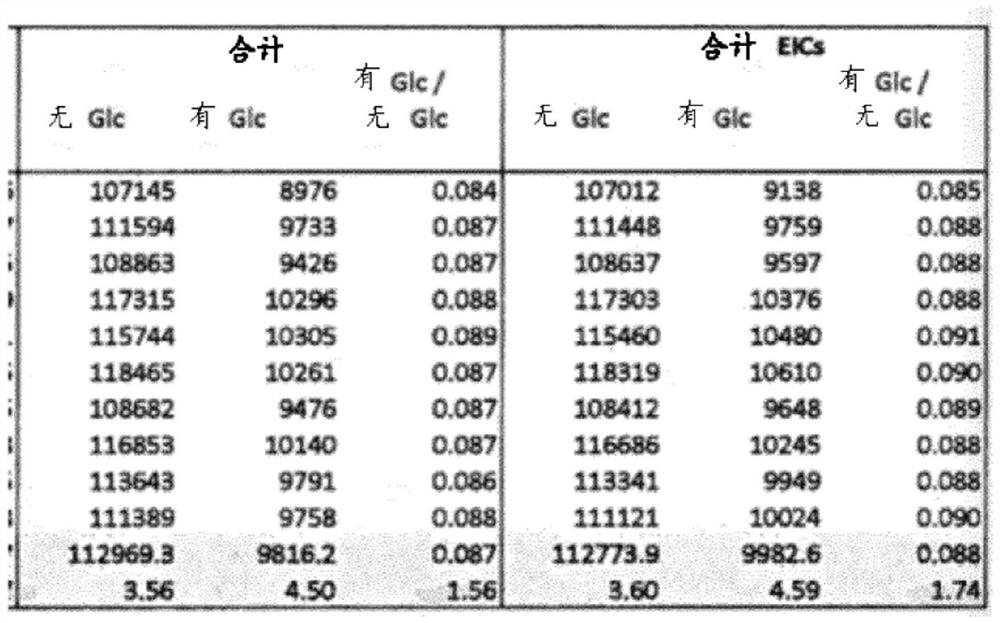
[0167] Determination of intact HbA1c in whole blood
[0168] Three 100 μL whole blood samples were each diluted with 400 μL deionized water and mixed by vortexing for 10 s until a clear solution was obtained. Then, each hemolysate was diluted 20-fold in different buffers with pH 2, 6 or 10, respectively.
[0169] Three batches of hydrophobic magnetic beads (100 μL per batch) were equilibrated by mixing with 900 μL of each of the three different buffers described above. Subsequently, 900 μL of the supernatant was discarded and the equilibrated beads recovered.
[0170] Three 900 μL samples of whole blood hemolysate (which had been diluted in three different buffers as indicated above) were added to the corresponding batch of equilibrated beads to obtain loaded beads. The mixture was then shaken at 37 °C and 1200 rpm for 15 min.
[0171] A series of several fractions for LC-MS were collected from the loaded beads as follows:
[0172] First, collect 900 μL of supernatant from...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com