Application of breviscapine as chemotherapeutic drug cardiotoxicity prevention and treatment drug
A technology of breviscapine and myocardial toxicity, which is applied in the application field of breviscapine as a chemotherapeutic drug for the prevention and treatment of myocardial toxicity. Improve myocardial damage and reduce oxidative stress
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
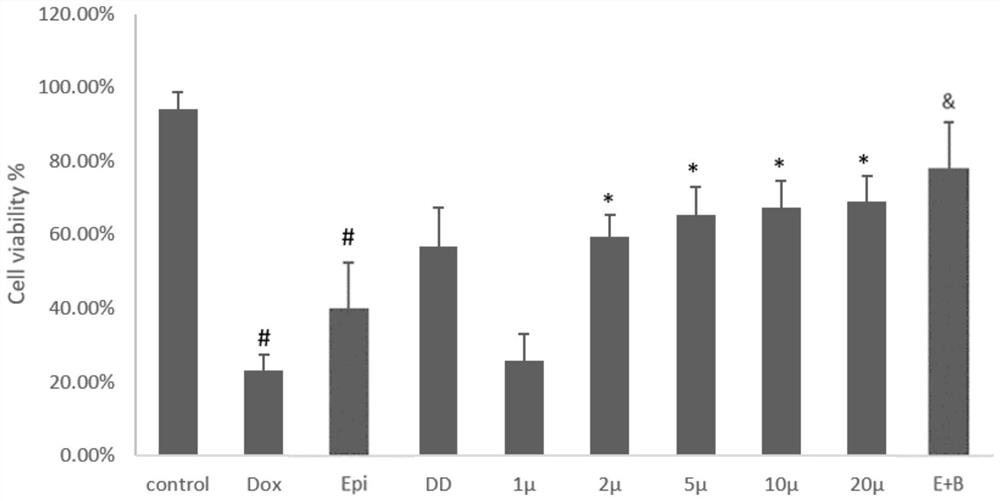
[0060] Example 1. Comparison of the effect of breviscapine on H9c2 cell activity by different anthracyclines (doxorubicin, epirubicin)
[0061] H9c2 cardiomyocytes were diluted with medium to 10 6 / ml of cell suspension, and inoculated in 96-well plates, adding 100 μL of cell suspension to each well, cultured for 24 hours, and then treated with drugs. Divided into blank control group (pure DMEM), model group 1 (5 μM DOX), model group 2 (5 μM EPI), positive control group (20 μM Dexra), 5 μM DOX+20 μM Dexra group, 5 μM DOX+Breviscapine group (breviscapine 10 / 20 / 50 / 100 / 200μM), 5μM EPI+Breviscapine group (breviscapine 200μM). After culturing for 24 hours, CCK-8 cell viability was used to measure the absorbance at 450 nm using a microplate reader to calculate the cardiomyocyte activity.
[0062] The result is as figure 1 As shown, compared with the control group cell activity of 94.08%, the 5 μM model group 1 cell activity was 23.11%, and the 5 μM model group 2 cell activity wa...
Embodiment 2
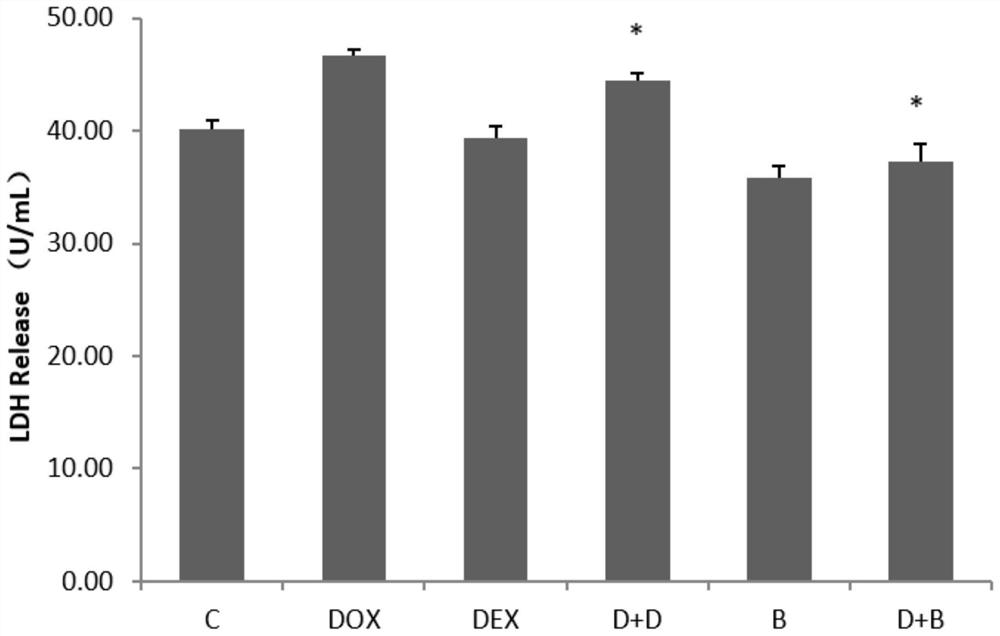
[0065] Example 2, the effect of breviscapine on reducing the leakage of lactate dehydrogenase (LDH) in adriamycin H9c2 cardiomyocytes
[0066] H9c2 cardiomyocytes were diluted with medium to 10 6 / ml of cell suspension, and inoculated in 6-well plates, adding 2ml of cell suspension to each well, cultured for 24h, and then treated with drugs. Set up a blank control group (pure DMEM), a model group (5 μM DOX), a positive control group (20 μM Dexra), and a DOX+Breviscapine group (5 μM DOX+200 μM Breviscapine). After culturing for 24 h, the LDH detection kit was used for determination. The results show( figure 2 ) The leakage rate of LDH in different medication groups was significantly lower than that in the model group.
Embodiment 3
[0067] Example 3, the effect of breviscapine on the level of superoxide dismutase (SOD) in adriamycin H9c2 cardiomyocytes
[0068] Superoxide anion (O 2-. ), O 2-. Nitroblue tetrazole can be reduced to form blue formazan, which absorbs at 560nm. The same treatment method was adopted as in Example 2. After culturing for 24 hours, the operation was performed according to the instructions of the CuZn / Mn-SOD activity detection kit. The results show( image 3 ), the DOX+Breviscapine group can significantly increase the activity of total SOD, which is significantly higher than that of the model group.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com