Methods and compositions for modulation of tau proteins
A composition, protein technology, applied in the field of regulation of Tau protein and composition, can solve the problem of not yet identified shortening or reversal therapeutic interventions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0189] Example 1: In vivo MAPT inhibition
[0190] A zinc finger protein for the MAPT(tau) target site as described in U.S. Publication No. 2018 / 0153921 was used as follows:
[0191] Table 1: MAPT-specific designs
[0192]
[0193] All ZFPs described herein are operably linked to the KRAB repression domain to form ZFP-TFs and all ZFP-TFs repress MAPT expression.
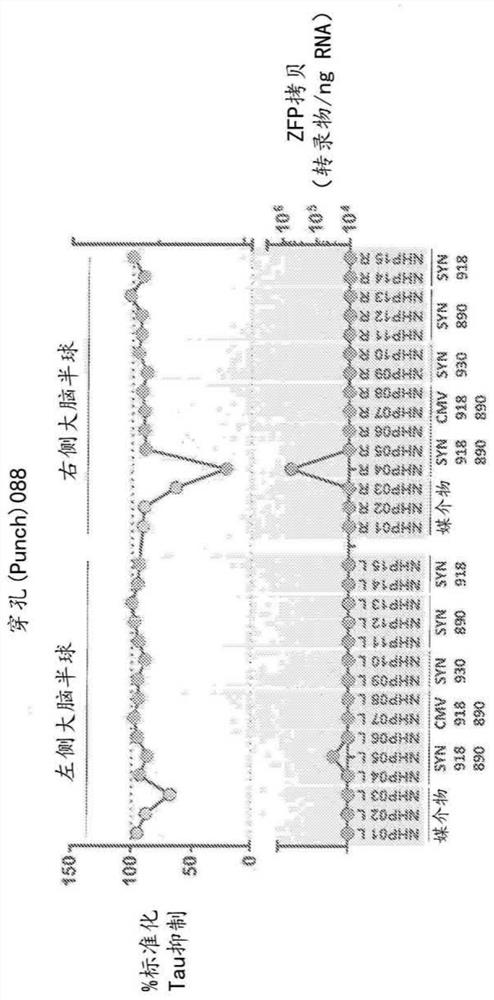
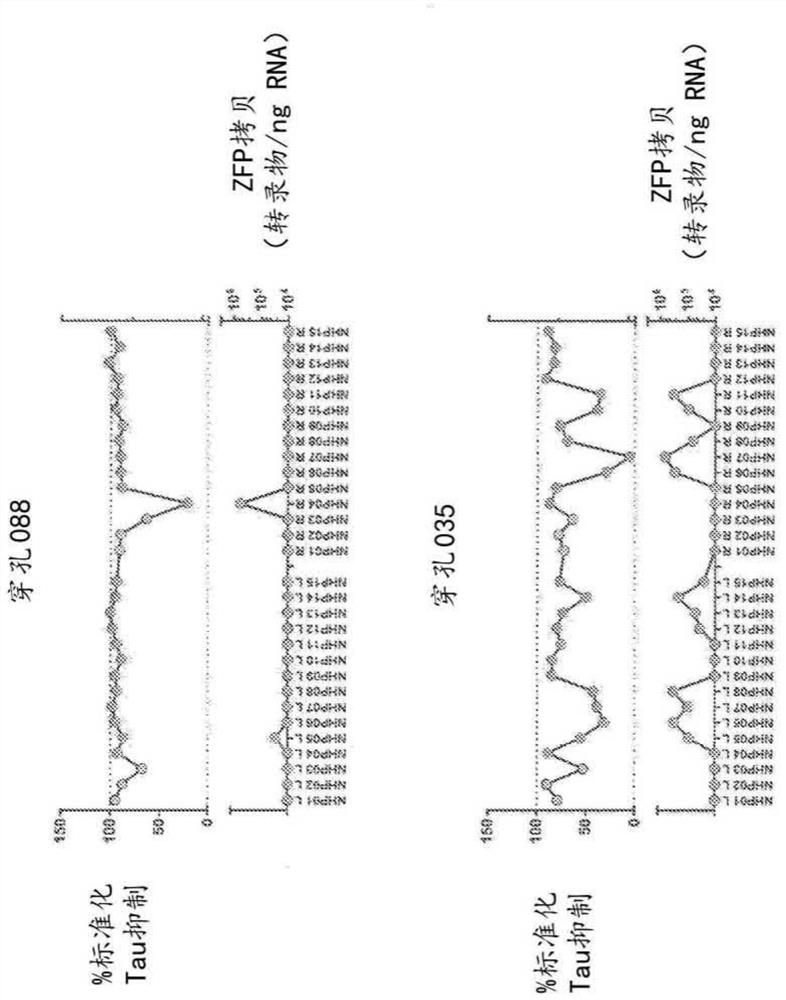
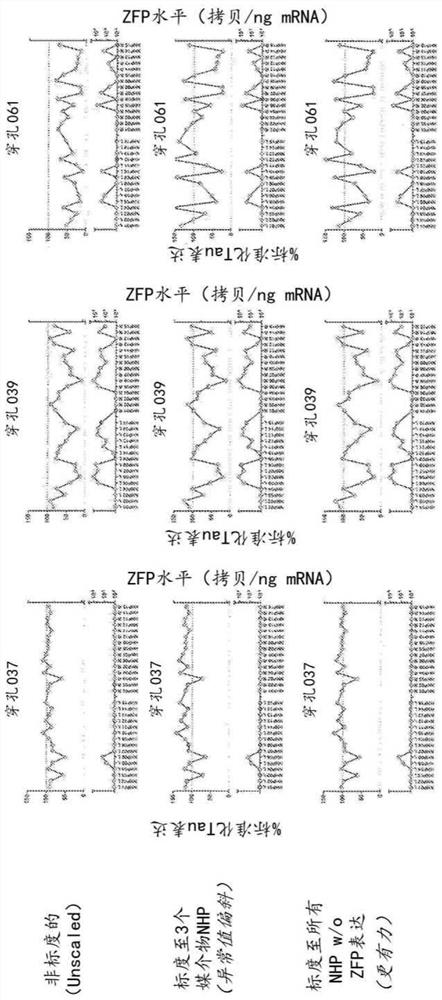
[0194] Primate tau-specific ZFP-TF was tested in cynomolgus monkeys (M. fascicularis) to observe tau expression in primates (non-human primate (NHP) model) suppression. Cynomolgus monkeys were housed in stainless steel cages equipped with an automatic watering system. The study complied with the Final Rules of the Animal Welfare Act Regulations (Code of Federal Regulations, Title 9) and the Guide for the Care and Use of Laboratory Animals. All applicable sections of the current edition of Laboratory Animals (Institute of Laboratory Animal Resources, Life Sciences Council, National Research Council, 8th ed.). ...
Embodiment 2
[0213] Example 2: Tau inhibition in a humanized tau mouse model
[0214] The P301L mutant human tau (P301L) transgenic mouse model (rTg4510, Jackson Labs) and the hTau mouse model (B6.Cg-Mapt tm1(EGFP )Klt Tau reduction in Tg(MAPT)8cPdav / J, Jackson Labs). hTau mice express the WT human MAPT gene, in addition, the endogenous (mouse) Mapt gene is knocked out and replaced with a GFP expression construct. The treatment groups are shown in the table below:
[0215]
[0216] *Administration route
[0217] ** Intraparenchymal (Ipa)
[0218] The endpoints measured are as follows: ZFP and Tau mRNA expression levels measured by RT-qPCR; Iba1, NeuN mRNA expression levels measured by RT-qPCR; Saitohin (STH) mRNA expression levels measured by RT-qPCR (STH is A protein-coding gene in apes and humans embedded in an intron between exons 9 and 10 of the human tau gene, see, e.g., Conrad et al. (2002) Proc Natl Acad Sci U S A.99 (11 ):7751-7756.); and tau protein levels.
[0219] like...
Embodiment 3
[0221] Example 3: Neuroinflammatory Response
[0222] Expression levels of microglial and astrocyte markers were also assessed in primates treated in vivo with the MAPT repressor ZFP-TF. Specifically, perforation as described in Example 1 was assessed using RT-qPCR reagents for IBA1 and GFAP expression. In addition, the levels of the E1F4A housekeeping gene were also assessed in treated primates. Briefly, brain punches were transferred to 1.5 ml Eppendorf tubes containing 0.6 ml TRI reagent (Thermo Fisher) and two 3.2 mm steel beads (BioPec products) on ice.
[0223] Tissues were lysed using a Qiagen TissueLyser at 4°C using the following parameters: 5 cycles for 90 s, frequency 25.1. After brief centrifugation, 70 μl of 1-bromo-3-chloropropane was added to each sample at room temperature. Vortex the samples for 10 s, centrifuge at 12,000 x g for 10 min at 4 °C, and transfer 120 μL of the aqueous phase from each sample to a well of a 96-well plate. 60 microliters of isopro...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com