Potential protein capable of serving as Alzheimer's disease drug action target and application thereof
A technology for Alzheimer's disease and drugs, applied in the field of biomedicine, can solve unclear problems and achieve the effect of weakened neural differentiation, significantly slowed down, and significantly reduced connections
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Example 1 Construction of AD cell model
[0060] (1) Construction of CRISPR / CAS9 gene editing method
[0061] The following processes are all operated under sterile conditions in the aseptic operating table:
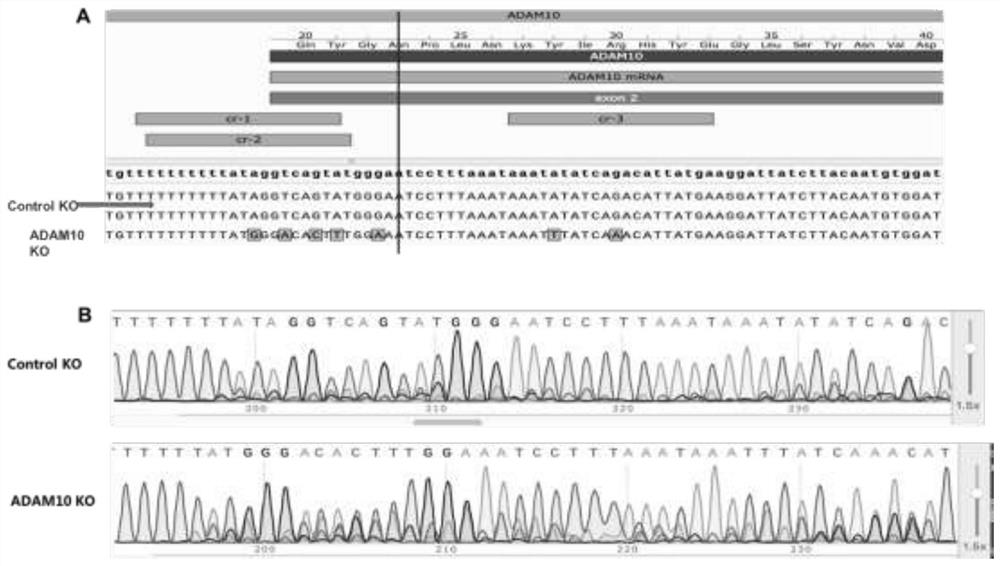
[0062] ① Construct the RNP complex targeting ADAM10 gene: take 0.33μl crRNA respectively ADAM10 1 (UUUUUUUUUUAUAGGUCAGUA (SEQ ID ON.1), 100 μM), 0.33 μl of crRNA ADAM10 2 (UUUUUUUUAUAUAGGUCAGUAU (SEQ ID ON.2), 100 μM), 0.33 μl of crRNA ADAM10 3 (AAAUAUAUCAGACAUUAUGA (SEQ ID ON.3), 100 μM) was thoroughly mixed with 1 μl Alt-R.CRISPR-Cas9tracrRNA (100 μM), and then diluted to a concentration of 20-80 μM with a nuclease-free buffer to obtain an RNA mixture. The RNA mixture was heated at 95°C for 5 min and cooled at room temperature for 10 min to obtain an annealed RNA mixture. Take 2.9 μl of the annealed RNA mixture and 1 μl of Cas 9 protein according to the ratio of 1.2:1-2:1, mix thoroughly and leave at room temperature for 10 minutes. After adding 0.6 μl elec...
Embodiment 2
[0094] 2.1 Determination of ADAM10 target protein and inflammatory body NLRP3 in AD cell model
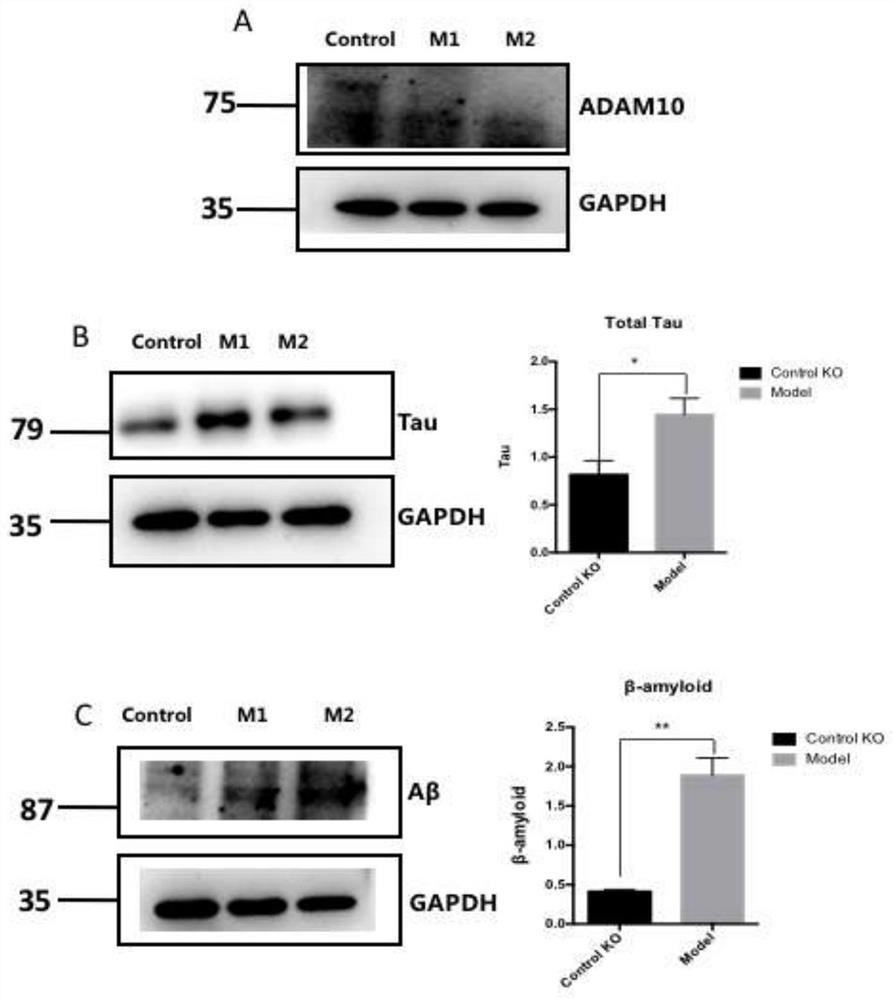
[0095] The protein level of ADAM10 in the knockout group was verified by Western staining. Such as figure 2 As shown in A, compared with the blank control KO group, the expression of ADAM10 protein in the ADAM10 KO group was extremely significantly decreased, which was consistent with the results of Sanger sequencing. The data further indicated that the ADAM10 gene was successfully knocked out in SH-SY5Y cells using CRISPR / Cas 9 gene editing technology. Subsequent experimental research can be carried out.
[0096] The inflammasome NLRP3 in the knockout group was verified at the protein level by Western staining. Such as image 3 As shown, compared with the Control KO group, ADAM10 KO group significantly increased NLRP3 and activated the expression of inflammasome.
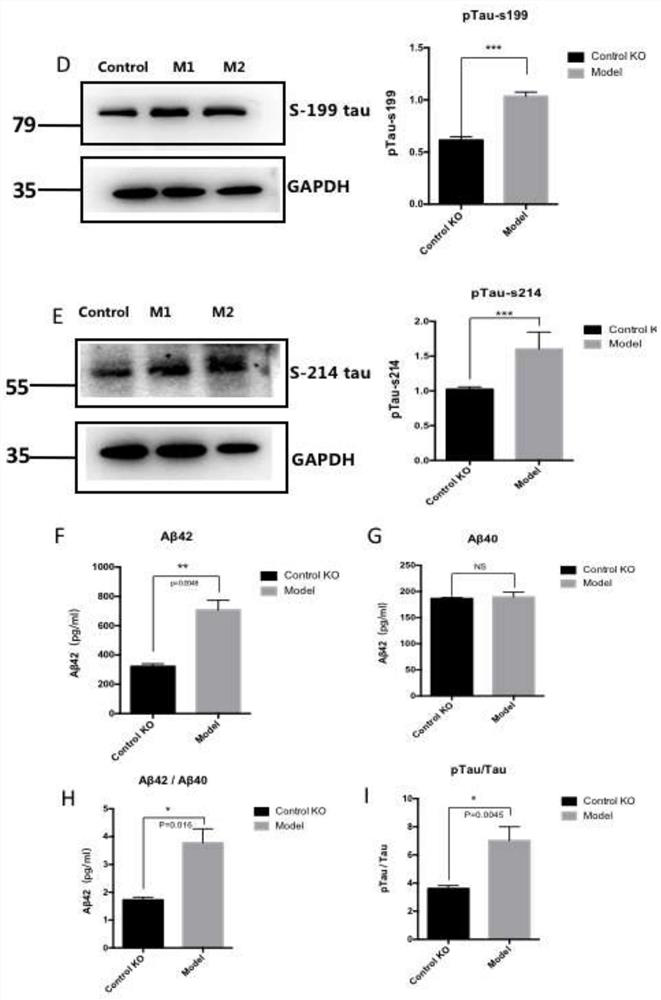
[0097] 2.2 Determination of Aβ and Tau protein in AD cell model
[0098] The accumulation of Aβ and intracellu...
Embodiment 3
[0111] Example 3 Constructing the knockout cell line of PTBP1
[0112] The following processes are all operated under sterile conditions in the aseptic operating table:
[0113] ①Construction of RNP complexes: Take 0.33 μl of crRNA1 (GGCACCCCCUUUUCAGCAAA (SEQ ID ON.4), 100 μM), 0.33 μl of crRNA2 (AAUGACAGCAAGAAGUUCAA (SEQ ID ON.5), 100 μM), and 0.33 μl of crRNA3 (AAAGGUGACAGCCGAAGUGC (SEQ ID ON.5), respectively. ON.6), 100 μM) and 1 μl of Alt-R.CRISPR-Cas9 tracrRNA (100 μM) were thoroughly mixed and then diluted with a nuclease-free buffer to a concentration of 20-80 μM to obtain an RNA mixture. The RNA mixture was heated at 95°C for 5 min and cooled at room temperature for 10 min to obtain an annealed RNA mixture. Take 2.9 μl of the annealed RNA mixture and 1 μl of Cas 9 protein according to the ratio of 1.2:1-2:1, mix thoroughly and leave at room temperature for 10 minutes. After adding 0.6 μl electroporation enhancement solution, react at room temperature for 5 minutes to...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com