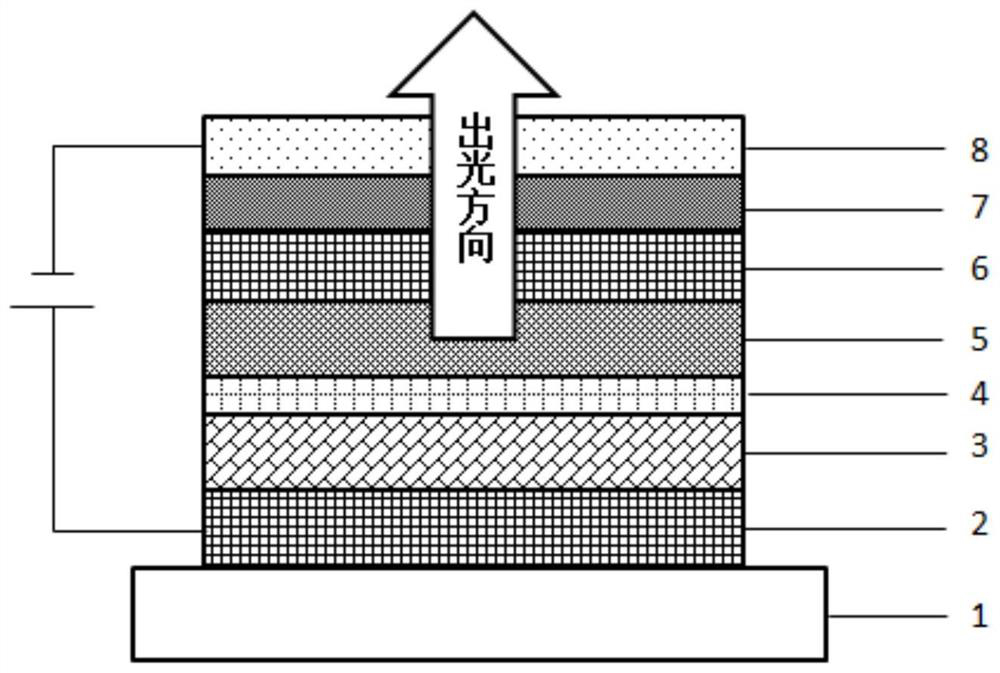
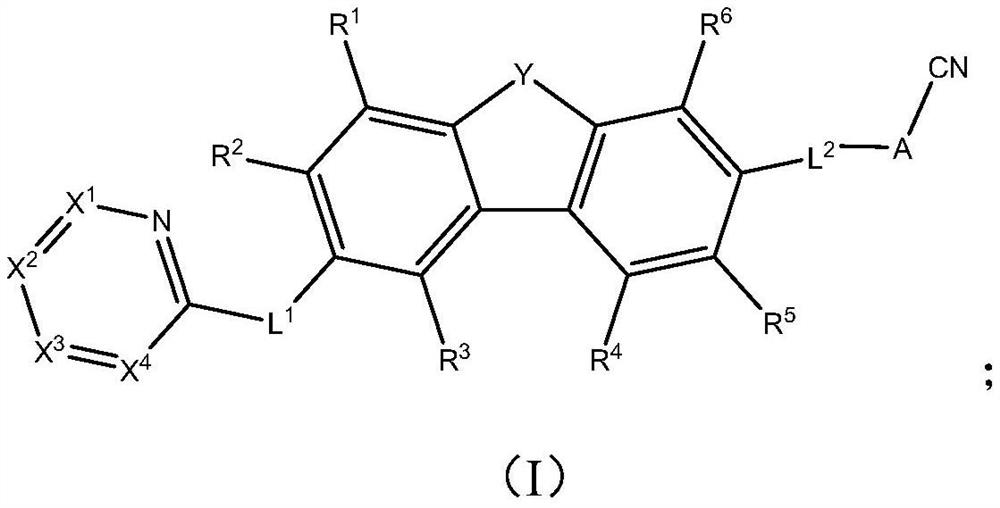
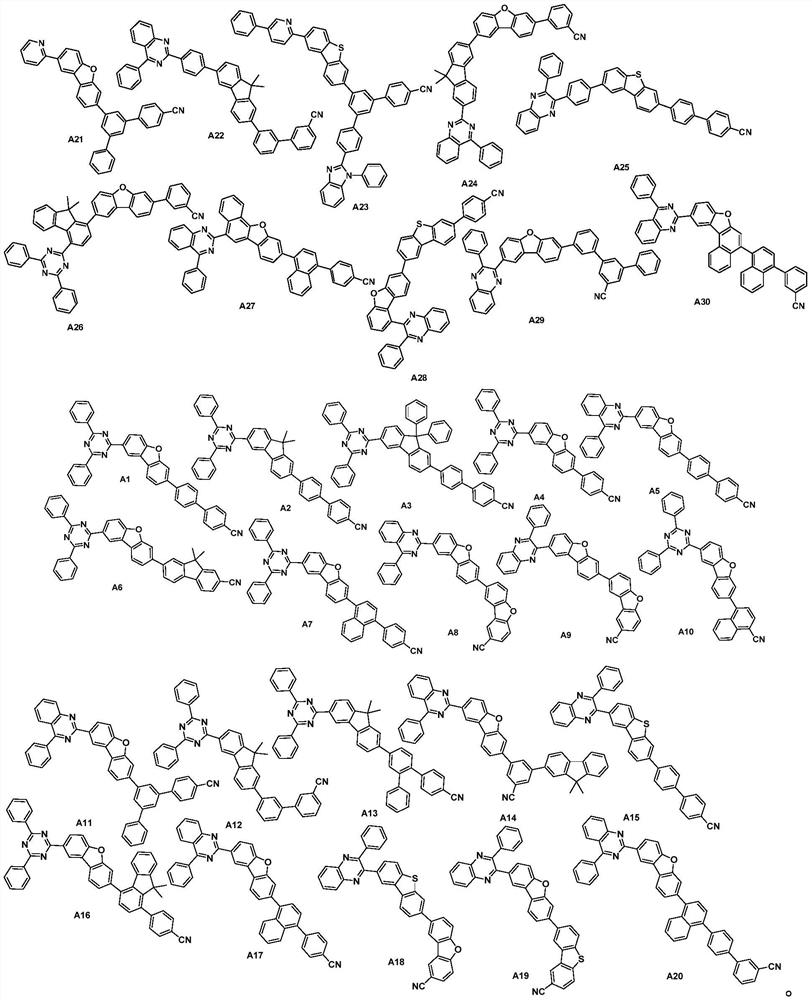
Aza-aromatic compound used as electron transport material and application thereof
A technology of aromatic compounds and heteroaryl groups, which is applied in the field of organic photoelectric materials, can solve the problems of OLED display device display function, low electron migration rate, and poor energy level matching that restrict OLED luminous efficiency, achieve excellent display effect, and simple preparation process Ease of application and life-prolonging effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0084] The synthesis of A1, the reaction process is as follows:
[0085]
[0086] The specific preparation process is as follows:
[0087] 100 mmol of 2-iodo-4-bromophenol, 100 mmol of 2-fluoro-4-chlorobenzene acid, 41.4 g of potassium carbonate (300 mmol), 800 ml of tetrahydrofuran (THF) and 200 ml of water, and 1 mol% Four (triphenylphosphine) palladium (PD (PPH) 3 ) 4 ). The reaction was reacted at 120 ° C for 12 h. After the reaction was completed, the reaction was stopped and the reaction was cooled to room temperature, add water, and the organic phase was concentrated to give a white solid, filtered, water wash, and the resulting solid was recombined with toluene to give a white powder M1. Among them, PD (PPH 3 ) 4 The addition of 1 mol% of 2-iodo-4-bromophenol was added.
[0088] 100 mmol of M1, 300 ml of dimethylformamide (DMF), 41.4 g of potassium carbonate (300 mmol), 120 ° C, reaction 12h. After the reaction is completed, add water, there is solid precipitation, filtere...
Embodiment 2
[0095] The synthesis of A6, the reaction process is as follows:
[0096]
[0097] The specific preparation process is as follows:
[0098] 100 mmol of 2-iodo-4-bromophenol, 100 mmol of 2-fluoro-4-chlorobenzene acid, 41.4 g of potassium carbonate (300 mmol), 800 ml of THF and 200 ml of water, and 1 mol% of PD ( PPH 3 ) 4 . The reaction was reacted at 120 ° C for 12 h. After the reaction was completed, the reaction was stopped and the reaction was cooled to room temperature, add water, and the organic phase was concentrated to give a white solid, filtered, water wash, and the resulting solid was recombined with toluene to give a white powder M1. Among them, PD (PPH 3 ) 4 The addition of 1 mol% of 2-iodo-4-bromophenol was added.
[0099] 100 mmol of M1, 300 mL of DMF, 41.4 g of potassium carbonate (300 mmol), 120 ° C were added to 120 ° C for 12 h. After the reaction is completed, add water, there is solid precipitation, filtered to obtain intermediate M2.
[0100] 100 mmol of M2, ...
Embodiment 3
[0107] The synthesis of A12, the reaction process is as follows:
[0108]
[0109] The specific preparation process is as follows:
[0110] 100 mmol of 2-boric acid-5-chlorobenzoate, 100 mmol of 3-bromide, 41.4 g of potassium carbonate (300 mmol), 800 ml of THF and 200 ml of water, and 1 mol% of PD (PPH) 3 ) 4 . The reaction was reacted at 120 ° C for 12 h. After the reaction was completed, the reaction was stopped and the reaction was cooled to room temperature, add water, and the organic phase was concentrated to give a white solid, filtered, water wash, and the resulting solid was recombined with toluene to give a white powder M1. Among them, PD (PPH 3 ) 4 The added amount of 1 mol% of 3-bromonol isryl.
[0111] 100 mmol of M1, 200 mL of THF, 0 ° C, 0.220 mmol of methyl bromide was added dropwise, and the drip was added dropwise to the room temperature reaction for 12 h. After the reaction is completed, water, the organic phase is separated, concentrated, and the intermediate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com